What do clinical stakeholders need to think about when wanting to ‘do digital’?

The opportunities for organisations and stakeholders using digital technology such as mHealth in the clinical setting are growing rapidly. Products such as Apple ResearchKit, Health Kit and Google Fit, and Microsoft’s HealthVault are all providing wider access to valuable consumer health data gathered from a variety of sources, including wearables and hardware sensors.
This article explores what technologies exist to enable mobile applications to interact with cloud servers and how these technologies can be deployed.
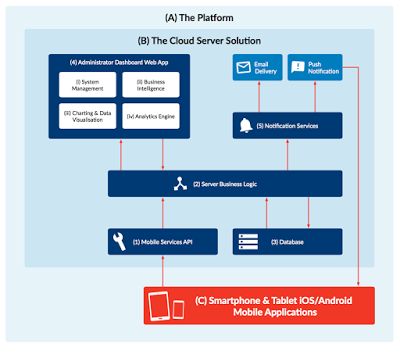
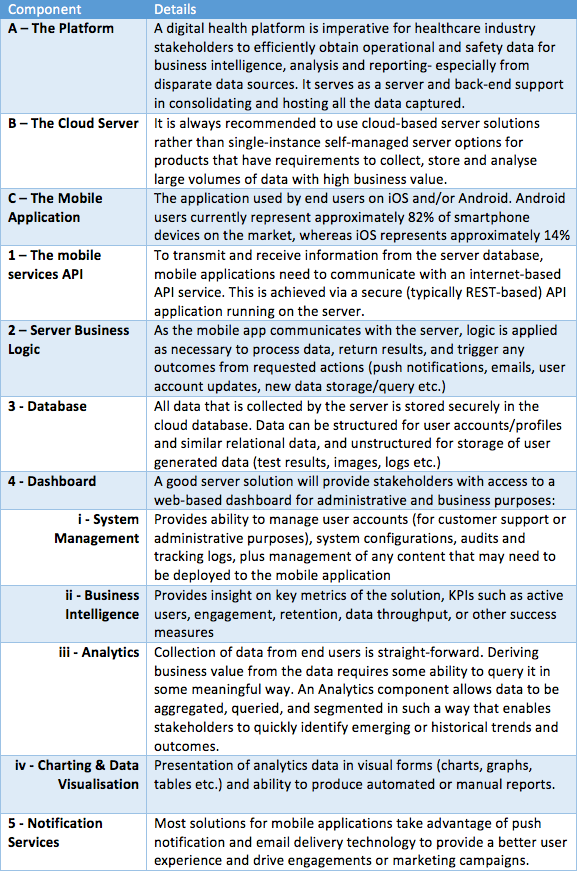
The diagram above shows the technologies driving digital adoption, followed by a table explaining each of the technology elements.
Digital health is a fast-growing and it has great potential to improve access and manage healthcare. Wide scale deployment and adoption also has the potential to improve efficiency, productivity and cost-effectiveness of healthcare delivery. There is great opportunity to transform the clinical trial sector and empower patients in the trial to take charge of their own health. The time to ‘go digital’ is now.
Sarah Iqbal is a research scientist with a background in biopharmaceuticals and business entrepreneurship. She is currently the Head of Digital Life Sciences at Biotaware Ltd, a connected health company with expertise in mobile app design and development, wearables integration and cloud server tech in clinical trials and consumer well-being industry.