Accelerating Alzheimer’s dementia research with collaborative research design

As part of Clinical Trials Digital Week earlier this year, Katrin Haeverans, Clinical Scientist and External Affairs Director, Janssen (EMEA) delivered a presentation introducing the European Prevention of Alzheimer’s Dementia Consortium (EPAD) and its revolutionary collaborative clinical trial design for Alzheimer’s dementia. Watch the full on-demand webinar here.
EPAD currently holds the largest ever public-private partnership in Alzheimer’s disease research. With 39 organizations across multiple disciplines, they are developing a research platform, in which partners across pharma, SMEs and academia will collaborate in Phase II clinical trials using subjects recruited from an established readiness cohort.
Challenges in Alzheimer’s dementia research
Haeverans starts by revealing the cruel truths about Alzheimer's dementia. In Alzheimer’s patients, the pathophysiological processes of dementia begin more than a decade before any identifiable symptoms, which has posed multiple obstacles in clinical research into the disease so far.
The consortium was set up in response to this, in a bid to accelerate research into Alzheimer’s dementia through collaborative study. Utilizing their collection of available resources, EPAD’s initiative is to alleviate:
- Difficulty finding the right test subjects due to the lateness of dementia symptoms in Alzheimer's
- 90% screen failure rate due to the misidentification of patients
- High costs of large phase III trials combined with insufficient use of adaptive designs
- Limited sensitivity of endpoints high intra- and interindividual variability, even more so if population is not homogenous
- RELATED ARTICLE: Innovative protocol designs in early phase clinical development - WHITEPAPER
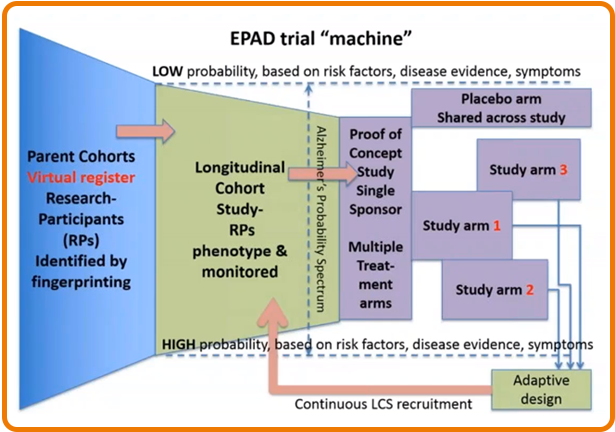
The Longitudinal Cohort Study
To drive the initiative, Haeverans explains, EPAD designed a longitudinal cohort study (LCS) to provide extensive investigation of patients’ eligibility for research. They developed a register, building onto existing patient registries across the dementia risk spectrum, from preclinical to mild dementia. This consolidated register will recruit eligible patients into a readiness cohort, where they will be phenotyped and studied over several years before entering the trial. The broad range of subjects available will also provide a wealth of collected run-in data.
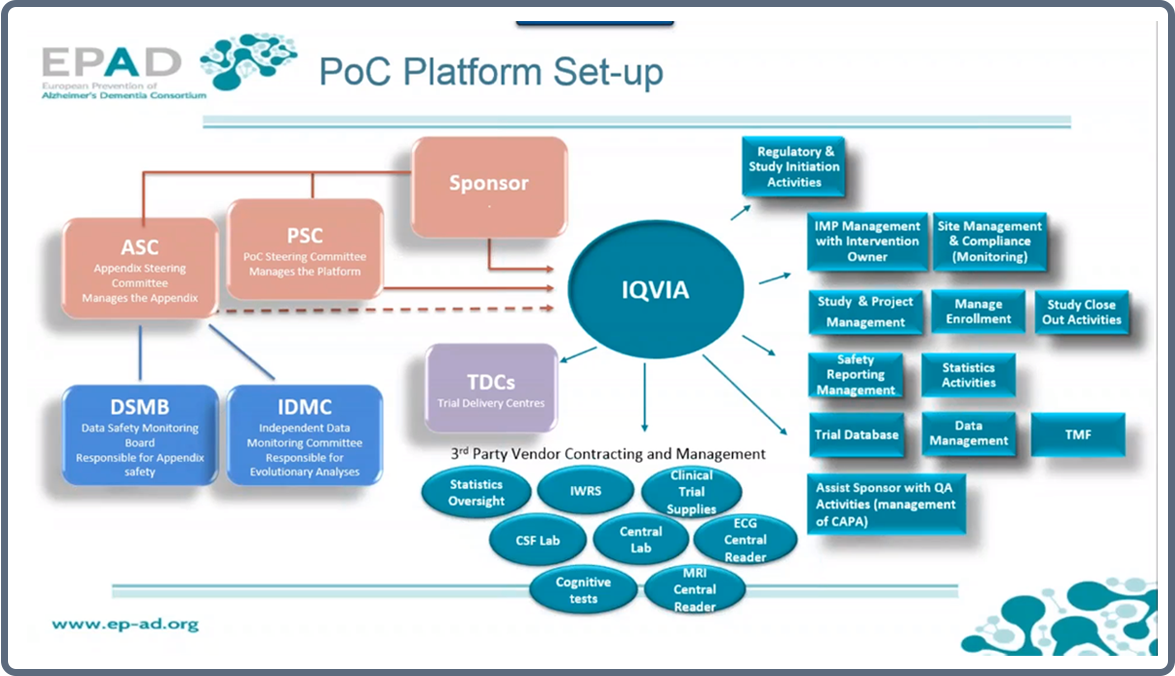
The PoC Platform
As Haeverans illustrates, EPAD’s research platform is based on a master PoC protocol, a common framework sponsored by the University of Edinburgh and shared between all partnering companies. Overseen by the Sponsor, the platform will be maintained and managed by a PoC Steering Committee.
For flexibility, each partnering company will additionally have their own appendix to the master protocol which will define the specifics of their own study arms of the platform. This will be governed by their Appendix Steering Committee, along with a Data Safety Monitoring Board, responsible for the safety within the Appendix, and an Independent Data Monitoring Committee which performs evolutionary analyses across all elements of the platform.
The clinical trial operations will then be outsourced to a CRO, IQVIA, who will manage the trials at EPAD’s 36 sites across Europe and third party vendors involved in the project.
The Intervention Owners
The LCS will feed pre-phenotyped patient subjects by equal randomization into each of the appendices, sorted at a ratio of three active treatments to one placebo.
Haeverans details how each appendix will provide a pharma company the flexibility of:
- Safety exclusions
- Subgroups enrolled
- Defining sample size, length of treatment and follow-up
- Randomization of sub-arms
- Biomarker analyses
As intervention owners, partnering companies will benefit from shorter timelines in research, a decrease in screen-failure rate, placebo sharing and access to rich data accumulated from the platform.