How mHealth, wearables and the Internet of Things will create the clinical trials of the future

The Internet of Things is increasingly a topic of conversation in the world of technology. To define it in very simple terms, IoT is a network of devices. This network connects any device, machine or sensor to the internet. Gartner forecasted that there will be 25 billion connected devices by 2020. So, it can be said that the IoT is a giant network of connected ‘things’, which indirectly includes connecting people because people use these ‘things’. As Jacob Morgan puts it in Forbes, this relationship will be between people-people, people-things, and things-things. The way all these devices work together is made possible by the fact that almost all of us now have access to the internet and a smartphone.
The birth of IoT was enabled by the internet, and more specifically broadband internet. Broadband internet is becoming more widely available and more devices (and now wearables) are created with Wi-Fi capabilities and various sensors built into them. In addition, smartphone penetration is increasing. More and more people rely on their smartphone (and other smart devices) to get online and go through their daily, even menial, tasks. In 2016, the number of smartphone users is forecast to reach 2.08 billion. By 2019, the number of mobile phone users in the world is expected to pass the 5 billion mark. These factors create the perfect environment for IoT, driven by the need of consumers to constantly be online.
Applications of IoT in Healthcare
IoT can be applied to many different industries including smart cities, transportation, and retail; healthcare isn’t an exception. In fact, healthcare is probably one of the best industries to apply IoT or IoMT - the Internet of Medical Things.
In the next five years, it is predicted that there will be a massive increase in IoT for the healthcare industry, also commonly known as the Internet of Medical Things. Experts predict that this sector will be worth $117 million by 2020. The IoT in healthcare market is segmented, based on application; patient monitoring, telehealth, medical and clinical trial operations, medication management, and connected imaging. Some key factors that are expected to drive the market include growing demand for real time disease management, improved patient care services and effective and efficient treatment outcomes.
The pharmaceutical industry has already started using various devices and wearables in many clinical trials to collect physiological data on trial subjects as these devices have the ability to collect better data continuously and improve overall patient experience. All of these form the basis of mHealth in the digital health industry.
The term ‘connected health’ is the term used to described how healthcare functions in the digital health industry. The “connected digital health industry” aims to maximise healthcare resources and provide flexible opportunities for patients to engage with clinicians and vice versa, with a goal of better self-care. IoT can be used to supplement patient treatment through remote monitoring and communication, and to keep track of a patient’s progress as they move through the healthcare chain.
IoT and clinical trials
Clinical trial complexity has increased due to geographical spread, more patients and more data to manage, and discrepancies in clinical investigators’ practices. Also, as the number of global clinical trials has increased in last few years, it has become more expensive, time consuming and taxing to monitor sites through onsite visits. With these issues comes the pressure of monitoring clinical trials effectively. The industry faces the challenge of making the drug development process more efficient; increasing productivity while reducing costs, and using appropriate technology to optimize the process is a large part of this. An IoT enabled system in a clinical trial is an innovative way to progress towards a solution.
The central idea of using IoT in clinical trials is remote monitoring thanks to fast data throughput. Through remote monitoring solutions, clinical trial administrators are able to extend trials more effectively outside dedicated testing facilities and into the homes of trial participants, delivering insights from patients in between visits to clinical trial sites, while reducing costs along the way. One thing to note however, is that on-site monitoring is still required by regulation for some critical study, though remote monitoring is seen as an acceptable addition to the process.
In a clinical trial, it is crucial to maintain subject safety and trial integrity. An IoT environment will enable healthcare professionals to monitor patients in the study more effectively, and use the data captured from the devices to assess any adverse events. In other words, by making the most of this network of devices, healthcare professionals are able to use data to create a continuous system of proactive management throughout the healthcare chain.
Remote monitoring in clinical trials should concentrate on activities that can be reviewed and monitored from a distance, such as data completeness from patients, discrepancy data or protocol violations at study sites. For example, where data is contained within the case report forms (CRF) or electronic records are added to an electronic trial master file (eTMF), an IoT system will enable access and therefore can be checked remotely for further analysis.
The Internet of ‘Medical’ Things enables clinical trials to focus on taking advantage of new opportunities to collect better data and improve the patient experience in a trial.
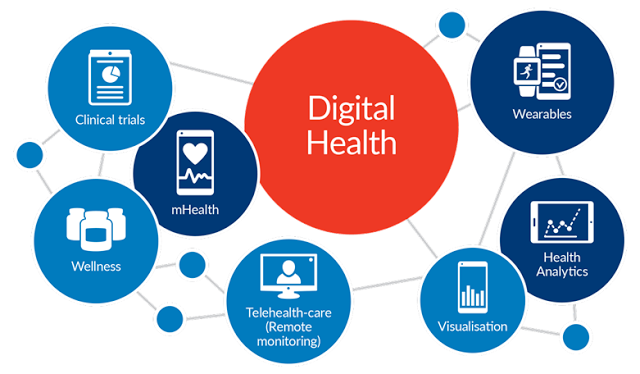
mHealth and wearables
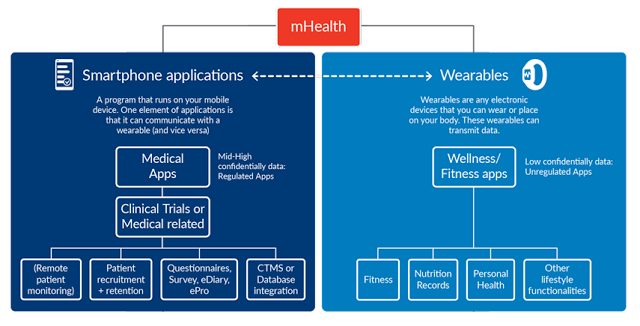
mHealth is a term used for the practice of medicine and consumer health supported by mobile devices. Mobile apps and devices can be used to deliver, access or record medical information, or provide clinical services (read our take on the mHealth industry here). mHealth gives patients and providers better access to essential health information at the correct time.
Another key development that is synonymous with mHealth application are wearables. Wearables (and sensors) allow daily activity monitoring and outcomes assessment in clinical trials. There are already hundreds of off-the-shelf sensors, wearables and mobile devices that can gather a stream of data from the day-to-day activities of participants and these efforts are already being progressed with companies like Pfizer and many others.
In an IoT network system, continuous data from a heart rate wearable, for example, can be transmitted from the patient’s home via WiFi or a smartphone to a data analysis server. Reports, which can include the walking speed and duration of each activity performed as well as energy used during exercise, can then be analysed almost instantly. For daily care, this readily accessible flow of real-world information allows clinicians to monitor the amount and (most importantly) quality of exercise for risk factor management and compliance. All of these can happen in real-time within an IoT system with the goal of having better participation (in form of engagement) from trial participants and more efficiency through connectivity.
Summary & Challenges
IoT in healthcare has enormous potential for the health and pharmaceutical industries. Proper implementation, integration and interoperability with reliable data repositories is crucial to enable this emerging opportunity towards better, more accurate and timely data in clinical trial research.
For clinical trials, an IoT system, with proper connectivity with devices, patients and study protocols allows for quality assessments of key activities, patient self-reporting, and enhanced patient engagement. This in turn can reduce the cost and burden of travel, improve patient recruitment and retention, and capture more reliable and valuable outcomes.
All this technology comes of course with its own technical and social challenges, ranging from data security and privacy, to compatibility between the various devices readily available on the market. These challenges must be overcome in order to ensure IoT adoption throughout the healthcare chain. More of these issues will be discussed in future posts.
About the author: Sarah Iqbal is Head of Digital Life Sciences at Biotaware Ltd, a connected health company with expertise in mobile app design and development, wearables integration and cloud server tech in clinical trials and fitness and lifestyle industry. She is based in Nottingham, UK.