Hype or hope? Can mHealth accelerate completion in clinical trials?

Clinical trials are the experimental foundation on which modern medicine is built. However, the painstaking process is costly, takes a great deal of time, demands intensive effort, and can have a very high failure rate. Reports from the FDA show that around 30% clear Phase I trials, 14% clear Phase II trials and only 9% pass through Phase III trials. The entire process can take anywhere from 6 to 10 years, which excludes time spent on preclinical drug research.
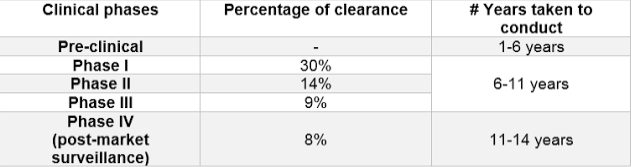 Breakdown of the clinical phases according to percentage of clearance and years taken to conduct the trial
Breakdown of the clinical phases according to percentage of clearance and years taken to conduct the trial
The cost of clinical trials
In 1975 the Tufts Center for the Study of Drug Development (CSDD) reported that the pharmaceuticals industry spent approximately $100 million in today’s dollars for research and development of the average drug approved by the FDA. By 1987, that figure had tripled to $300 million. By 2005, this figure had more than quadrupled to $1.3 billion. The true amount that companies spend per drug approval is almost certainly even larger today.
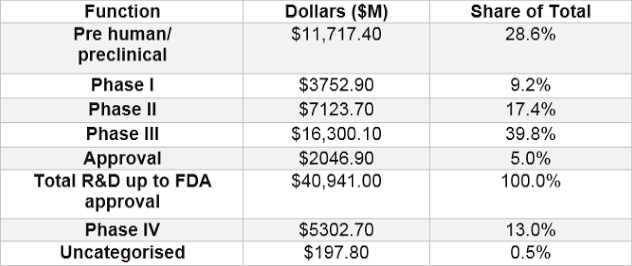
 The Pharmaceutical Research and Manufacturers of America (PhRMA) reported that the total cost of Phase I-III research has increased by 250% since 2001 – to slightly over $40 billion. The table below shows a breakdown of the estimated cost for each clinical phase incurred while developing a drug.
The Pharmaceutical Research and Manufacturers of America (PhRMA) reported that the total cost of Phase I-III research has increased by 250% since 2001 – to slightly over $40 billion. The table below shows a breakdown of the estimated cost for each clinical phase incurred while developing a drug.
Over the years, the amount of clinical trial research for pharmaceutical and biological products has increased globally. All this research and development yielded an extensive amount of new drugs, making it increasingly difficult for pharmaceutical companies to prove their products are superior to those already on the market. Clinical trial development is becoming ever more complex, specifically from a regulatory perspective, with greater emphasis on data and site/patient monitoring. The need to accumulate more data to show modest benefits has resulted in drug companies investing more in recruitment for clinical trials, which also further increases the cost.
Since clinical trials contribute the biggest cost percentage in drug development, it is clear this needs to be cut. Reducing the duration and cost of clinical trials increases the potential to make profit on a drug, whilst also driving down the cost of treatment, and ultimately improving outcomes for patients.
Strategies for reducing cost already include offshore trials and outsourcing, but mobile health is increasingly playing a role in trial efficiency. Technology, and particularly mobile technology, should always be considered as an option to streamline clinical trial processes and studies have shown mHealth can reduce cost. So, is mHealth the default solution for accelerating completion in clinical trials?
Using mHealth in recruitment
The number one cause of delayed completion, and therefore increased cost, is due to patient recruitment and retention. Patient accrual in clinical trials relies heavily on effective recruitment, but enrolment goals are not met for a variety of reasons. No matter the cause, delays in clinical trials affect sponsors, CROs, and sites.
There are various initiatives and organisations that have been set up to offer patient and volunteer recruitment to clinical trial sponsors and some CROs claim to have a high rate of patient recruitment. Yet despite this, it still remains a major issue. Why is this still happening?
One contributing factor is the failure to track recruitment efforts. It is important to ask each subject how they heard about the study and then document the information and how it is obtained. Sites can use metrics and analytics, with mHealth offering hard data, to justify why they need an increased advertising budget for example. Examining what was effective and what did not work helps plan future recruitment, knowing what to continue doing and to avoid making the same mistakes. Integrating mHealth with patient recruitment websites can help track such information to innovate the recruitment process.
Engaging patients with mHealth & wearables
Patient engagement is key when addressing the issue of retention. A growing body of evidence demonstrates that patients who are more actively involved in their healthcare experience better outcomes and incur lower costs. Patient engagement is a concept that combines patient activities with interventions designed to increase activation and promote positive patient behavior, such as obtaining preventive care or exercising regularly. Patient engagement is a strategy to achieve the ‘triple aim’ of improved health outcomes, better patient care, and lower costs.
 The emergence of wearable technology and the popularity of patient-centric applications for them provides a great opportunity for pharmaceutical manufacturers to engage patients and create a customised, direct relationship with them. The integration of wearable and mobile technology in new clinical trial design and business strategy development holds promise for aligning site and patient needs with faster study execution and reduced costs.
The emergence of wearable technology and the popularity of patient-centric applications for them provides a great opportunity for pharmaceutical manufacturers to engage patients and create a customised, direct relationship with them. The integration of wearable and mobile technology in new clinical trial design and business strategy development holds promise for aligning site and patient needs with faster study execution and reduced costs.
Using a wearable tech in trials, like a handheld electrocardiograph or a blood regulator microchip patch, and consolidating the data collected via an app, can streamline the process. The biometrics collected from the device can be especially useful for trials conducted on subjects that have two or more chronic conditions like diabetes and a heart attack, or patients with degenerative diseases like Alzheimer’s, as tracking and measuring data from them (people who need a long-term commitment to measuring and tracking their health) would be useful for pre- and post-clinical trial studies.
mHealth in a lean approach
In the entrepreneurial world, the sooner a failure can be identified, the more productive time can be devoted to successful ventures. Because of the high failure rate in clinical trials, adopting the lean method is increasingly stressed as a way to reduce costs. If you are to fail, fail at the early stages.
Using digital and mHealth can facilitate this lean method in clinical trials as real-time data enables earlier visibility of side-effects and risks. So if the drug is not working, this will become evident sooner, allowing earlier failure, and the ability to pivot to a different solution, thus reducing cost.
Because mHealth has been shown to be able to streamline the clinical trial process, pharmaceutical companies realise the benefits of mHealth, hence they are even putting aside special budgets for their pilot projects in mHealth. Pharma giants such as Novartis, Pfizer, Astra Zeneca and GlaxoSmithKline are betting big on leveraging mHealth technologies thought in the drug development process, so we should expect the increased use of mobile health tools in clinical trials in the near future. This goes to show that pharmaceutical companies already believe in the importance of using mobile technology in the drug development process, specifically for patient engagement, and hence the mHealth industry is projected to grow even further.
Hype or hope?
It has been demonstrated that integrating mHealth in clinical trials can reduce cost. However, are these cost savings enough to make any significant impact revenue-wise in the long term? The challenges that mHealth has to overcome in the industry are primarily around regulations, so working closely within the regulatory landscape will likely be the best way to fully leverage mHealth.
To capture mHealth’s full potential, industry stakeholders will need to act in a coordinated fashion to redesign their approach in streamlining the clinical trial process. Working with a trusted technology solutions provider and developer that has the experience and capability in medical deployment is also key to a successful deployment.
The hype of leveraging mHealth in clinical trials has stirred some drastic movements within the industry. Ultimately, the drug development industry will either choose to go digital or be forced to. Earlier adopters of digital may already be realising the future potential that mHealth has to offer in cost reduction and streamlining the clinical research process. The stragglers may be more established, enabling them to compete for longer with their old models, but sooner or later all players will be adopting digital.
Sarah Iqbal is currently the Head of Digital Life Sciences at Biotaware Ltd, a connected health company with expertise in mobile app design and development, wearables integration and cloud server tech in clinical trials and fitness and lifestyle industry. She is based in Nottingham, England, UK.