Implementing the ICH E6 R2 guidelines and operational risk based approaches to oversight

The introduction and implementation of ICH E6 R2 has raised a lot of questions around operational risk based approaches to oversight. Both sponsors and CROs are working to understand and interpret the guidelines, as well as overcome a range of challenges.
We asked Patricia Leuchten, Founder and CEO of The Avoca Group, about these issues and got an exclusive early look at Avoca’s 2017 Report on Industry Approaches to Risk Assessment and Management. The report is based on research from 394 participants across Sponsor and Provider organisations.
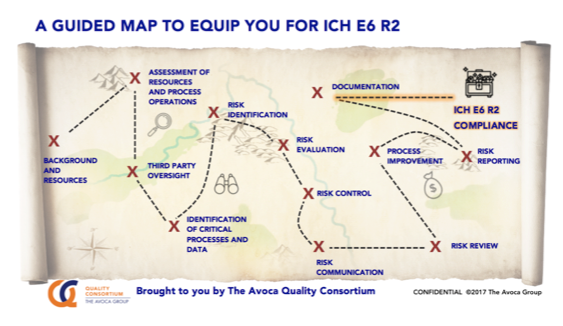
For further support, download the AQC ICH E6 R2 guidance and road map of tools.
How should we as an industry interpret and put into action the ICH E6 R2 guidelines?
PL: “The ICH E6 R2 guidelines make risk-based approaches to quality management mandatory and require these to be documented as part of a GCP Quality Management System. Interpreting the guidelines is not the challenge – they are more prescriptive than the previous version of ICH E6. However, many companies are finding that operationalizing the approaches that are stipulated by the guidelines requires both a shift in practice and a shift in mindset.
Compounding the challenge is that many companies report gaps in understanding how to implement risk-based approaches within their organizations and across partnerships. This is quantified in the report.”
The report states that:
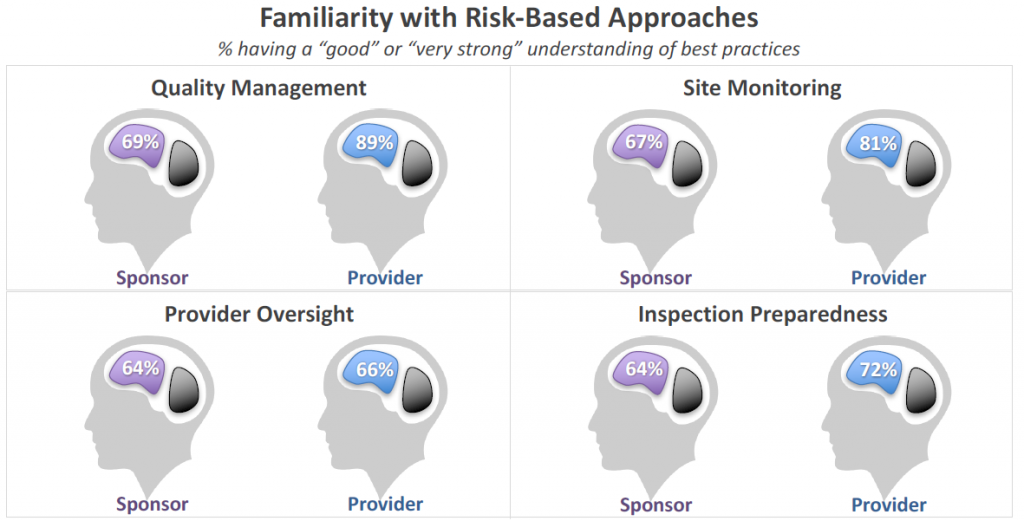
Approximately a third of Sponsor respondents indicated only fair knowledge to no understanding of best practices in risk-based approaches. Providers expressed greater understanding overall, however they were on par with Sponsors for understanding of risk-based approaches to Provider Oversight.
PL: “The AQC has mapped industry leading practices to the ICH E6 R2 requirements to enable companies to rapidly identify practices and tools that will prepare them to satisfy new guidelines.”
What are the biggest challenges to successful implementation?
Only about one-third of respondents indicate that gaining alignment on risk is “easy” from an internal perspective. Even fewer say this is the case when attempting to gain alignment with external parties. - Avoca 2017 Report on Industry Approaches to Risk Assessment and Management
PL: “The biggest challenges are related to having to develop and implement approaches that may be unfamiliar to operational staff members and that require prospective thinking and risk prevention. These approaches typically require a change in corporate culture and a shift in behaviors. For example, historical behaviors are centered around addressing current challenges. The shift that is required is a laser focus on preventing future risks from becoming tomorrow's issues. Making this shift is the biggest challenge that organizations face. Organizations would benefit from having processes and tools that drive the required prospective behaviors.”
The report includes a number of quotes from respondents highlighting specific challenges they are facing and the actions being taken to tackle them:
- Aligning/Formalizing Processes & Tools: “We've had challenges in bringing together disparate data sources into one RBM view point. We've also been applying RBM approaches to legacy studies where the study setup has not been conducive to data integration.”
“Introduction of new risk management SOP and associated templates, updates of other numerous SOPs which are impacted by this approach.” - Aligning People & Thinking: “Mind shift - understanding that implementing QRM and RBM is not about saving money. Teams still report issues, not risks and the risks identified are mainly focusing on timeline and budget, not on quality."
“Adaptation of such an approach and the buy-in from all functional parties. They don't understand it well enough to adopt and allow the needed resources to be applied in order to get it off the ground.” - Training: “Train clinical teams to understand risk assessment approaches for proactively reducing risk.”
- Focus on Oversight: “Moving to RBM and more fully embracing a risk-based approach to managing trials and vendors, and also insisting that CRO partners use this approach also.”
- Implementing/Improving QMS: “We are creating an Integrated Quality Management Plan for the company that will formalize many of the risk management processes that we currently perform in a more informal fashion. Additionally, we have created a clinical Quality Management Council that will be able to review risks and issues in an ongoing manner.”
What factors should be considered when assessing an organization’s readiness?
PL: "Organizations should conduct a gap analysis to understand where they may be deficient in the following areas that are mandated by ICH E6 R2:"