MHealth in clinical trials: 79% of companies increasing usage - data report analysis

KNect365 recently conducted one of the biggest surveys of its kind of clinical trial professionals asking about the most hyped areas of clinical trials today and whether they are actually impacting the way studies are run. 199 clinical trial professionals from pharma and biotechs (24%), CROs (23%), service providers (21%), consultancies (11%), sites (6%), academia (5%) and patient groups (2%) completed the survey, largely split between North America (55%) and Europe (33%).
A large section of the survey explored the perceptions, implementation, benefits and challenges of mHealth, AI and data technologies. Respondents made it clear that they think these are vital to the future of clinical trials, with 53% placing mHealth or AI as having the biggest impact on clinical trials by 2030. Here we dive into this data to pick out key trends of where the industry is currently at and where it is going with the technology revolution in clinical trials.
Download the full Beyond the Hype in Clinical Trials industry report here.
Implementing mHealth technologies
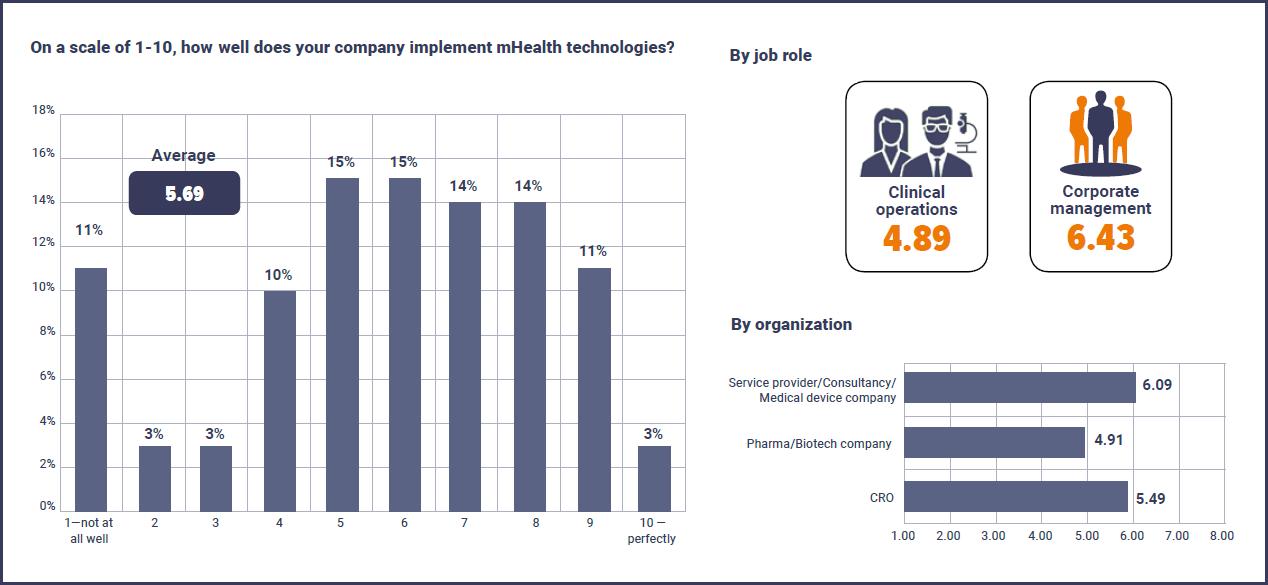
MHealth technologies are increasingly changing the way clinical trials are run and this is backed up by 79% of respondents who said it was ‘Likely’ or ‘Very Likely’ their company will increase its use of mHealth in the next two years. However, clinical trials professionals clearly think there is still a lot of work to be done with current integration of this technology. When asked to rate how well their company is currently implementing mHealth technologies on a scale of 1-10 (1 being ‘not at all well’ and 10 being ‘perfectly’), the average score was just 5.69/10. Furthermore, 11% of respondents gave the lowest score of 1/10 in how well their company is implementing mHealth technologies, versus just 3% who said their company was implementing mHealth perfectly.
 Interestingly, a split was seen between corporate management respondents who gave an average rating of 6.43 and those working in clinical operations who averaged just 4.89. Also, perhaps unsurprisingly, respondents from service providers and medical device companies feel they are implementing these technologies better than pharma and biotech companies with an average of 6.09 versus 4.91, with CROs sitting in between the two on 5.49.
Interestingly, a split was seen between corporate management respondents who gave an average rating of 6.43 and those working in clinical operations who averaged just 4.89. Also, perhaps unsurprisingly, respondents from service providers and medical device companies feel they are implementing these technologies better than pharma and biotech companies with an average of 6.09 versus 4.91, with CROs sitting in between the two on 5.49.
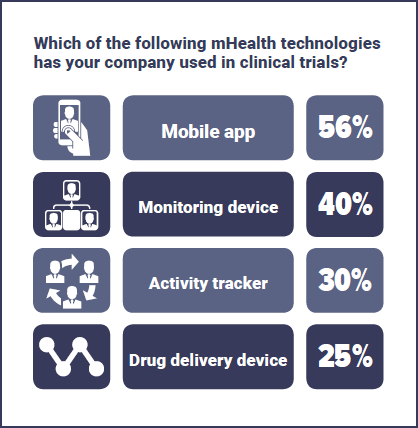
Of those currently implementing mHealth, mobile apps were the most common form of technology with 56% of respondents, followed by monitoring devices (40%) and activity trackers (30%). Other responses included ePRO, mECG, sleep measurement, voice-based technology, time and date stamp medicine adherence, eLabel, SMS reminders, video observation and “multi-modal communications tailored to patients' preferences”.
The benefits and challenges of mHealth technologies
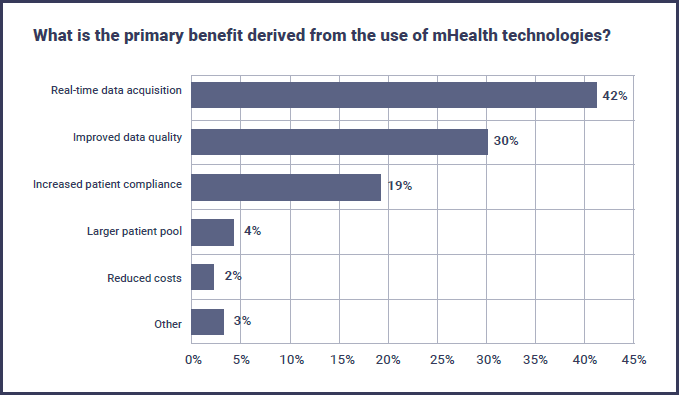
There are a variety of potential benefits to using mHealth technologies, but the majority of respondents focused on data generation as the primary advantage, with 42% citing real-time data acquisition and a further 30% on improved data quality.
With the proliferation of clinical data generated by new technologies, it can often seem that companies are drowning in Big Data. However, our survey respondents suggested that in general companies are managing and utilising the data well – giving an average score of 7.27 when asked to rank out of 10.
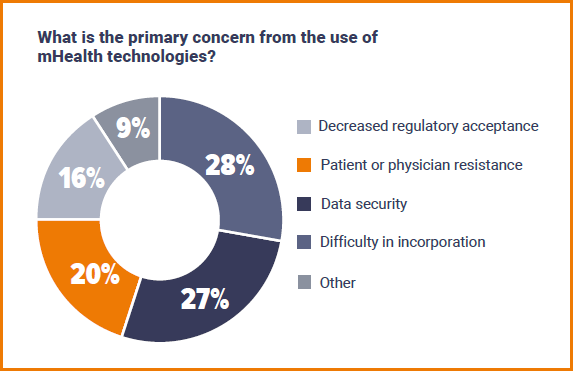
On the other side of things, when asked about the primary concern around mHealth, answers were split quite evenly between difficultly in implementation (28%), data security (27%) and patient or physician resistance (20%). Whilst respondents were happy to choose one of the provided options for the benefits of mHealth tech, it is telling that they were far more vocal when given the opportunity to discuss concerns. Answers included:
 - “Lack of data standards used by mHealth companies to help integrate data with a central database.”
- “Lack of data standards used by mHealth companies to help integrate data with a central database.”
- “Unless you nail the patient to the floor there will ALWAYS be problems with the use of more and more intricate and involved e-technologies that will result in bad or skewed data, or bad relations with clinicians / sites and the sponsor, who puts it on the consultant to 'correct' somehow.”
- “Organizational resistance”
- “Systems don't work well. Poor design.”
- “Slow industry adoption”
- “Sponsors all have different methods for using such technologies. What must be understood is that they don't always work or there are difficulties implementing them with patients. Computerised methods look great on paper, but when they just don't work because patients are unable to utilise them appropriately, or circumstances occur which prevent their appropriate use, crucial data is not collected and this causes no end of difficulties for site staff / consultants trying to collect this data. Some of these technologies are just too complicated and 'iffy' to work in all instances.”
- “Cost”
- “Ease of use for the patient.”
- “Increased time the site has to spend training employees and patients on the tool; troubleshooting and monitoring the compliance of the subject.”
- “When data is not 'optimal', sponsors then pressure consultants and clinical staff to 'make' it work, often causing friction between all parties resulting in ongoing bad feeling.”
It’s clear that there are still a number of concerns with the adoption of mHealth, yet the importance of the varying technologies to clinical trials isn’t in doubt. In the report introduction, Citeline succinctly sums up the industry’s perception:
“Despite difficulties in incorporating mHealth into trials as well as data security, evidence supporting adoption of mHealth products, such as mobile apps and activity trackers, continues to grow, paving the way for this technology to realize key benefits, including real-time data acquisition and improved data quality.”
Artificial Intelligence in clinical trials
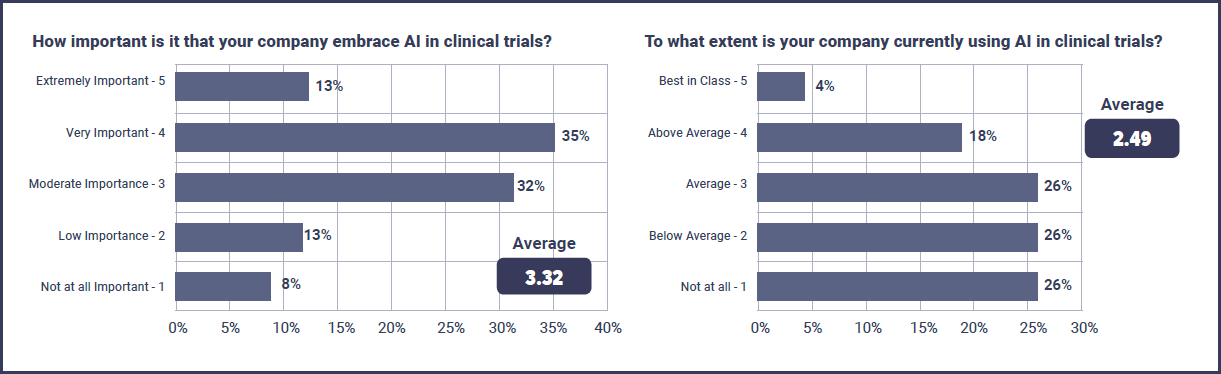
AI is still in the early stages of being incorporated into clinical trials and this is reflected by the fact that just 22% of respondents believed their company was ‘above average’ or ‘best in class’ against 52% who saw their company as ‘below average’ or not using AI at all.
However, whilst AI may not be implemented all that widely, the importance of it to clinical trials in the future isn’t doubted, with 80% of respondents saying it is moderately to extremely important that their company embraces AI.