Outsourcing in clinical trials: an EU struggle? - Data report analysis

In the collaborative environment of clinical trials, partnerships and outsourcing are essential, yet can open the door to a world of day-to-day challenges. In our recent State of the Industry Report 2019, we investigated what today’s clinical trials professionals see as the most prominent risks faced in managing relationships with third-party organisations.
The survey was completed by 214 professionals across CROs (29%), pharma and biotech (28%), consultancies, medical device companies and technology providers (26%) investigator sites (6%) and academia (4%). The result was a glimpse of current perspectives around partnerships and outsourcing, from regulatory changes to struggles with vendor oversight.
Download the full State of the Industry Report 2019 here.
Challenges with vendor oversight and governance
Oversight of vendors is one of the most stressful challenges within clinical trial management, adding potential threats to various aspects within clinical trials. However, frustration with vendor oversight appears to be more prominent in Europe, as 63% of European respondents said they found the process either ‘Quite’ or ‘Very’ challenging, opposed to only 47% of those in North America.

Adherence to timelines had the highest selection by respondents as the biggest challenge in vendor oversight and governance, while cost management and effectiveness ranked second.
Some may argue that working with overly ambitious targets and short budgets is to blame for this. Interestingly, regulatory compliance showed to have the least concern while outranked by quality of execution and data. Selected responses include:
- Being able to oversee all vendor activities with limited internal resources. Regarding the governance, it is not always easy to have access to key decision-makers on the vendor's side.
- Vendors are too focused on their own processes and ways of working and are poorly adapted to client requests.
- Different SOPs lead to misunderstandings in processes, complicated agreement approval process for vendors in Russia due to sanctions.
- Ensuring an overall view of safety data. Being capable to quickly react and take appropriate actions to a safety issue that could arise related to an IMP in all ongoing CT (of the same or outsourced sponsors) at the same time.
- Deciding on hands-on responsibility in case of issues - who is taking the decision to act and does the action needs approval by sponsor?
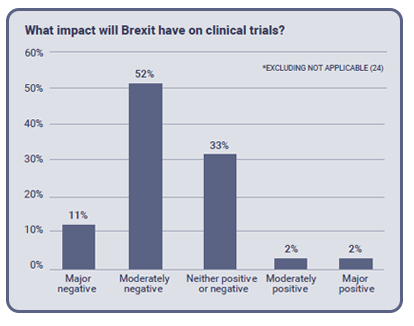
The impact of Brexit on clinical trials
Unsurprisingly, the current status of Brexit negotiations has encouraged reservations among clinical trials professionals. 63% of our respondents said they expect that Britain’s departure from the EU would have a negative impact on clinical trials. Only 6% said they believed the process would have a positive impact, and a third of the respondents said they were unsure about what the effect could be. This reflects the uncertainty within the situation, and as the negotiations take shape, perspectives within the industry are likely to change.
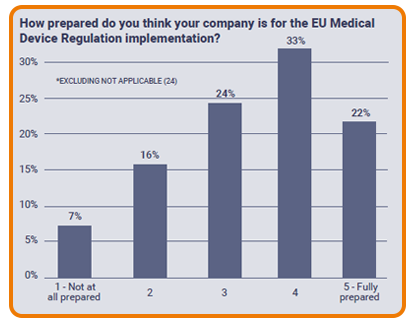
Preparing for implementation of the EU MDR
More than half of European respondents expressed confidence about their companies’ preparations for implementation of the new EU Medical Device Regulation. While 22% said they were fully prepared, a third of the respondents said their companies were almost there. More concerningly, a quarter of respondents said their organisations were either less or not at all prepared for implementing the EU MDR, indicating a thirst for guidance remaining within the industry.
Download the full State of the Industry Report 2019 here.
RELATED ARTICLE: Clinical Trial Vendor Oversight and Governance - WHITEPAPER