The past, present and future of wearable technology in clinical research

The intersection of technology and healthcare covers a range of solutions in various sectors of the digital health industry. One particular solution is wearable technology. While healthcare organizations such as pharmaceuticals in clinical trials have not yet embraced wearables in large numbers, they are certainly intrigued. Wearables are emerging as a solution for creating more efficient clinical trial processes, from recruiting patients to collecting real-time patient data.
Mobile health (mHealth) tools that enable clinicians and researchers to monitor the type, quantity, and quality of everyday activities of trial participants have long been needed to improve daily care, design more clinically meaningful randomized trials, and establish cost-effective, evidence-based practices. Wearables (or activity trackers), also seen as a mobile health tool, have begun to fulfil this need.
The birth of wearables- how it all started
Wearable technology, in the simplest terms, can be defined as items such as jewellery, glasses, watches or clothing that is worn on or around the body and incorporates sensors and other electronic technologies - think Bluetooth Wi-Fi, gyroscopes and so on. Wearable technology has been around for far longer than most people think; it can be traced back to the 19th century, as this brilliant infographic of the history of wearables shows.
 The evolution of wearable technology (Source: Racounteur)
The evolution of wearable technology (Source: Racounteur)
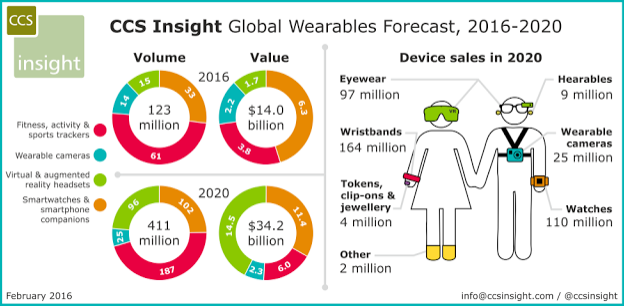
Wearable tech is currently going through rapid growth, with analysts claiming the industry will hit USD $14 billion in 2016. The driving force behind this, is the advancement in hardware, in terms of practicality, affordability and functionality.
Global Wearable Forecast 2016-2020 (Source: Forbes)
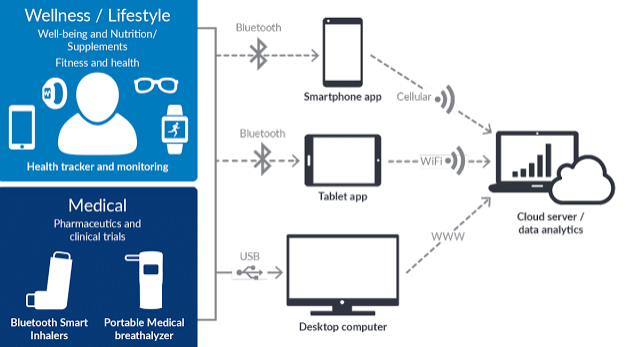
Fitness and healthcare applications that promote healthier living and lifestyle have emerged as the front-runners in the adoption of wearable technology. However, the features and focus of these wearable will go well beyond just fitness. There are a number of clinical trials that have begun to incorporate wearables or other digitally enhanced devices into their studies.
Applications of wearables in the fitness and clinical research context (Source: Biotaware)
Wearables in a clinical trial setting
Clinical trials have increased in complexity over a number of years through geographical spread, increased numbers of patients, more data to manage and discrepancies in clinical investigators’ practices. Due to the nature of clinical trials these complexities probably won’t decrease and hence, there is a need to manage them. Wearables are emerging as one of the solutions that combats these issues throughout the clinical trial chain. When applied to clinical trials, wearable technology can be a powerful research tool to gather data, therefore enabling trials to progress more smoothly.
The central idea of using wearables in clinical trials is using remote-monitoring technology to monitor the participants in the trial and the physiological data that comes from them. Sensors that can continuously monitor the user’s vital signs, motor activity, doctor-patient and social interaction, sleep patterns and other health indicators. This data can then compliment other types of data captured, for example through Quality of Life (QoL) questionnaires. Use of wearables obviously depends on the therapy area being investigated, but these technologies are versatile and can be applied to most medical research.
How data is transmitted from wearables
It is important to note that most wearable health devices transmit data via an app and therefore work well only in conjunction with software. Gartner forecasted that wearables will drive 50% of all app interaction by 2017. Continuous data from wearables can be transmitted from the home via Wi-Fi, through smartphone apps or to a remote data analysis server. These activity trackers are good examples of the Internet of ‘Medical’ Things and how it is used in the connected digital health industry.
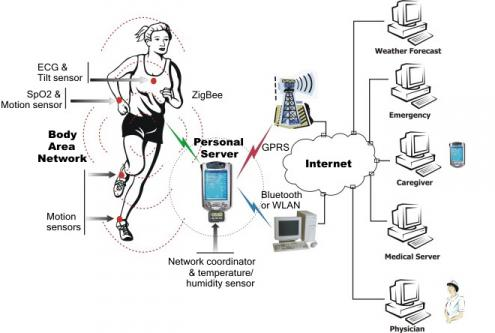
Wearable technology also includes the Wireless Body Area Network (WBAN) - a wireless network of wearable computing devices. A WBAN can include a number of physiological sensors depending on the end-user application. BAN devices may be implanted inside the body, be surface-mounted on the body or be accompanied devices humans can carry in pockets or bags.
Wire Body Area Network (Source: Waves)
How wearables can help
According to a 2015 SCORR/Applied Clinical Trials survey of CROs and other service providers, mHealth’s greatest benefits will come from improving data accuracy and patient experience. Wearables working in parallel with other mHealth tools could offer a number of benefits:
- - Data generated by wearable devices can be used by pharmaceutical and life science companies to run more robust clinical trials and capture data to support outcome-based reimbursement.
- - The huge expense of clinical trials can limit the size and sensitivity of drug testing, and harmful interactions are sometimes detected only months or years after a drug is introduced to the general population. Wearable tech will enable early detection of abnormal conditions from physiological data and prevent any serious consequence.
- - If life science companies can get enough insight early in development, they can potentially create a more efficient drug development process and prioritize resources for the most promising therapies, thus getting effective drugs to market faster and more safely. Early safety signal detection not only leads to better patient protection, but also has the potential to save development costs.
- - Progressing through a clinical trial can be taxing for patients who feel communication is restricted with their clinician or feel left out during the research process. One of wearable tech’s core functionalities is interpersonal communication supported by continuous biomedical sensing. Communication is critical in the clinical trial context as patient engagement is synonymous with compliance.
- - Wearables allow for patient engagement through gamification, which can be done through a patient’s personal circle or social media. For example reinforcing an individual’s social support system may be the most effective way to encourage not only compliance towards the clinical research, but also adopting healthier behaviour patterns.
How to move forward?
Clinical research adoption of wearable tech will depend on ease of use, relevance and accuracy. There are currently a number of limitations for wider acceptance of wearables including:
a) Lack of system integration of individual sensors
b) Interference on a wireless communication channel shared by multiple devices
c) Non-existent support for massive data collection and knowledge discovery
To move forwards a number of important steps must be taken:
Create standards: Standards must be created within the context of the attempted outcomes assessment.
Accuracy and Precision: Wearables need to be deemed accurate or precise for medical assessment (which is indirectly tied to creating standards).
Integration, interoperability, connectivity and security: Hardware (wearables themselves) and software (apps, servers and back-end systems) need to smoothly integrate and interoperate. In addition, there needs to be constant connection continuity between apps, wearables and the internet to ensure no data is lost when captured.
As new sensors and communication technology becomes available, healthcare professionals will be able to organize huge medical databases for use in tracking every test taken and medicine prescribed over an individual’s lifetime. This will result in a lot of data. Data analysts need to be trained to evaluate and identify the data pertinent to trial outcomes, as stakeholders, patients and consumers place greater value on companies that can help them use data to improve their health.
Finally, creating such an information architecture of course requires safeguards to maintain the privacy of the individual and any stakeholders.
Summary
As the clinical trial industry transitions from passive to active safety surveillance activities, there will be greater demand for more comprehensive and innovative approaches that apply quantitative methods to accumulating data from all sources, ranging from discovery and preclinical, through to clinical and post-approval stages. As wearable technology becomes cheaper and more sophisticated, and data quality improves, these devices and their associated apps will become a part of patient and consumers’ lives and the health ecosystem.
At Partnerships in Clinical Trials Europe, a whole stream was devoted to disruptive innovation in clinical trials, including IoT and wearables. Over 1000 industry leaders gathered in Vienna - find out more here.
About the author: Sarah Iqbal is a business scientist with a background in biopharmaceuticals and business entrepreneurship. She is active in the intersection of technology and healthcare, focused on building mobile and digital applications for healthcare and clinical trials. She strives in the efforts to digitise healthcare, simplify clinical processes and streamline communications between stakeholders, clinicians and patients within the industry. She is currently the Head of Digital Life Sciences at Biotaware Ltd, a connected health company with expertise in mobile app design and development, wearables integration and cloud server tech in clinical trials and fitness and lifestyle industry. She is based in Nottingham, England, UK.