Assessing the impact of patient-centric initiatives on clinical trials with Tufts CSDD - ON DEMAND WEBINAR

Stella Stergiopoulos from the Tufts Center for the Study of Drug Development presents data from their study on patient-centric initiatives. Watch the full webinar presentation, as well as six other on-demand webinars here.
Regulatory agencies, biopharmaceutical companies, patient advocacy groups and patients are all collaborating to create more patient-friendly and centric research. However, there has been very little data collected on the prevalence of specific patient-centric initiatives or their impact.
The Tufts Center for the Study of Drug Development, in collaboration with the Drug Information Association and many biopharmaceutical partners, has conducted a comprehensive study that explores the current prevalence of the use of patient-centric initiatives as well as assessing the initial impact these initiatives have had on study performance.
The results of this study and their implications were presented as part of PCT Digital Week by Stella Stergiopoulos, Research Fellow and Senior Research Project Manager at Tufts CSDD.
Her presentation starts by looking more generally at the current trends in patient-centricity across the industry, before honing in on the work that Tufts CSDD has done.
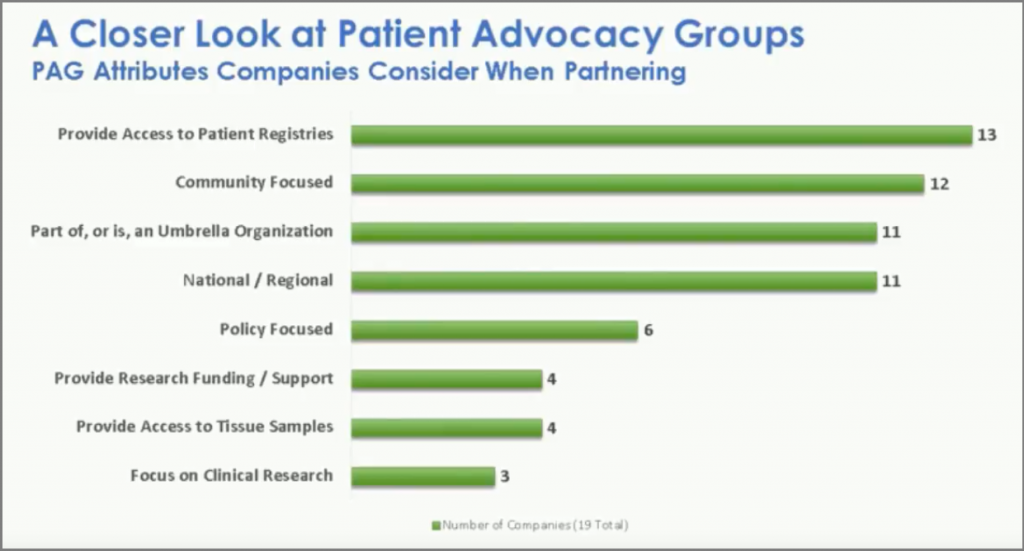
Part of the presentation involves a closer look at the benefits of partnering with a patient advocacy group, though, as the table above shows, Stergiopoulos points out that 'not all groups are born equal'.