REPORT: The 8 biggest challenges facing clinical trial professionals

As with any industry, the challenges facing those working in clinical trials at the moment are numerous; from patient recruitment, to adoption of technology, and regulatory requirements, to spiralling costs.
Over the last year we have asked dozens of clinical professionals representing pharma, CROs, sites and vendors about the biggest challenges they are facing and the graph below shows their most common responses. We delve into the top replies to discover what it is about these issues that are most challenging trials today.
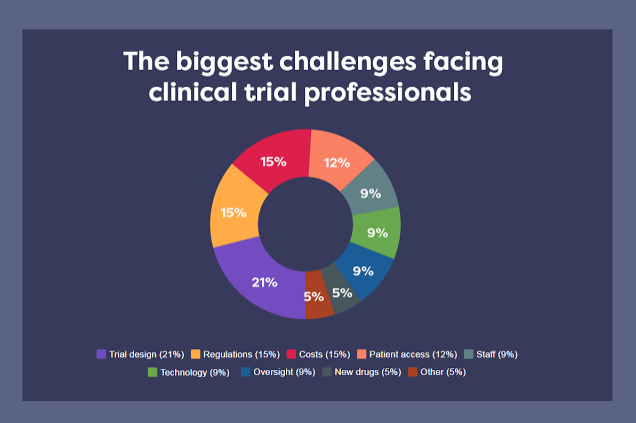
1. Complexity of Trials (21% of respondents)
Clinical trials have been growing increasingly complex for years, and those running them are feeling the pressure to design trials ‘that give the right answers, in the most simple and unobtrusive way for patients, that are acceptable to regulators and payers’.
Respondents from study sites are particularly concerned with meeting the ‘challenge of focussing on the best study design’ for ‘very complex modern clinical trials’. Such concerns are borne out in the ‘high rate of failure to meet primary endpoints due to poor or complex design’.
2. Regulations (15% )
A predictable concern for an industry so heavily regulated, many respondents feel constrained by the complexity of the guidelines they follow. Additionally, the variations between different regulatory bodies, and the challenges that brings, comes up repeatedly.
As trials are increasingly looking to emerging markets in new countries, there is also the need to understand whole new sets of requirements.
3. Spiralling Costs (15%)
An inevitable result of the above two issues, the cost of trials is at an all-time high. Increasing complexity and tight timelines is putting more pressure on the ‘need for resources to implement and control every step’.
4. Patient access (12%)
Patient recruitment and retention are a major challenge for those running trials, with a high percentage not meeting targets and drop-out rates increasing. One respondent blamed the ‘increased burden for patients through study participation without adequate “return of investment” in form of personal benefits’. Such concerns explain the prevalence of patient centric approaches in today’s research.
5. Staff Roles & Responsibilities (9%)
As the complexity and rate of change in trials both continue to increase, hiring and training the right staff is getting equally tricky. Respondents commented that ‘working in a constantly dynamic environment both in and out of the company’ means that ‘roles and responsibilities are evolving’.
Another aspect is the ‘increasingly remote nature of teams’ which has ‘an impact on how we manage and retain staff’. Similarly, with trials becoming more and more geographically diverse, another new challenge is ‘obtaining experienced clinical research professionals in developing countries’.
6. Technology (9%)
Technology is already playing a massive role in improving many aspects of trials and is rightly seen as one of the great hopes for future. However, with it, there are a whole raft of new challenges facing those running, participating in and regulating trials. As one respondent put it: ‘With technology, so much is happening so fast, the challenge is how to select and use the RIGHT technology, and for that technology to gain the acceptance of patients, healthcare professionals and regulators.’
7. Governance and oversight (9%)
Strategic partnerships, vendors, study sites, CROs; partnerships are playing a more important role in almost every aspect of trials today and the management of them can often prove challenging.
From the perspective of a study site, one particular challenge is the ‘involvement of a huge number of vendors in the studies’.
8. New drugs (5%)
Brand new classes of drug require different ways of running trials. Cell therapies, genomics, personalised treatments and whatever ‘the next big breakthrough is’ were all mentioned by the respondents as creating new issues in ‘demonstrating clinical effectiveness’.