Analytical tools to improve decision-making during antibody-drug conjugate development
Speed-to-clinic and then speed-to-market are highly significant metrics for companies developing biopharmaceuticals. By increasing the pace of drug development, firms reduce costs, obtain revenues earlier and can establish commanding positions in the market relative to competitors. High throughput development tools have contributed much to the acceleration of drug development. They allow the testing of a large number of process parameters and when combined with multi-factorial experiments, enable the design of highly efficient and well-characterised bioprocesses.
One challenge with adopting such an approach is that the bottleneck shifts from the process development department to the analytical testing laboratory. Small biotech companies often face a dilemma with respect to the analysis of samples from early stage experiments. Either they must purchase expensive, sophisticated analytical technologies and employ the necessary human resources to operate these effectively or they must outsource to contract analytical laboratories often increasing the time taken to obtain results.
The biopharmaceutical industry is short of cost-effective analytical tools that allow rapid decision-making during the development process that help them navigate their way to the clinic and then beyond.
Case Study: Antibody-drug conjugate development
The development of antibody-drug conjugate (ADCs) processes illustrates this point. Process development scientists often wish to screen a large number of conditions during the conjugation step to identify those that will deliver an optimum drug-antibody ratio (DAR). Development scientists can perform the experiments relatively quickly and at small-scale to screen different methods of conjugation with a range of solvents. How then can scientists ensure the conditions are giving a quality product?
An effective ADC drug must first bind to its target receptor, be internalized by the cell and then release its cytotoxic payload into the cell cytoplasm. End-point, cell-based assays will show whether these three steps are occurring effectively but are often only accessible by small to medium biotech companies through contract research organizations. For this reason, many choose to make assessments on the quality of the product based on a molecular analysis of the conjugate. This provides some information such as the DAR ratio but does not provide information on functionality. A reliance on a molecular analysis alone could be misleading. Identifying conditions that give high DAR ratios to optimize loading are of no use if high toxophore loading leads to impaired antibody binding.
Development engineers in the ADC industry need a method for analysing ADC products during process development that allows the timely assessment of ADC functionality without recourse to costly cell-based methods. Orla Protein Technologies Ltd worked with Glythera Ltd., an emerging biotechnology company focused on the development of antibody-based treatments for cancer as well as broader based therapeutics. They have developed a fusion protein consisting of a surface binding unit and the Her-2 domain IV receptor for trastuzumab using Orla’s OrlaSURF protein engineering technology (ref 1). This allows anchoring of the fusion protein to surfaces while controlling the structure and orientation of the target protein so that its natural structural milieu is mimicked.
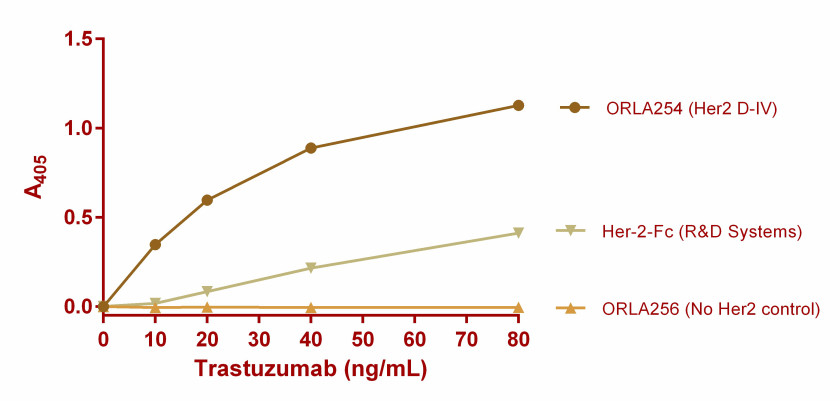
The OrlaSURF technology gives a more sensitive assay compared to absorbed native Her-2 protein because of the superior way in which the receptor is presented (Figure 1). The technique can be transferred to analytical biosensors for rapid, real-time, high throughput analysis (ref 2). The technique is applicable to not only ADCs, but also to a range of biologics including antibodies and vaccines.
ELISA showing Trastuzumab binding to ORLA254, control SBU (ORLA256) and native Her-2-Fc fusion produced in eukaryotic cells (R&D Systems). Average data from three wells plotted. The display of Domain IV on OrlaSURF results in greater sensitivity of the assay compared to adsorbed native Her-2 protein. Limit of Detection <10ng/mL on OrlaSURF Vs 20 ng/mL for native protein. The lower detection limit means that much less precious ADC needs to be used in the analysis.
Analytical methods for strategic advantage?
It would be a mistake to underestimate the importance of having an optimized approach to analytics during the development of biopharmaceutical products. Executives can only make effective business decisions if they have the correct information about the molecule in development. This applies across the biopharmaceutical industry. For example, in the race to develop biosimilars, being able to assess comparability to the innovator molecule during development enables managers to gauge the likelihood of a successful commercialization at each stage in the development cycle. Understanding that a biologic in development may not have the necessary quality attributes allows firms to take the difficult, if necessary, decision to terminate the project and focus their resources on alternative programs with greater opportunities for success.
Biopharmaceutical analytical technology is a field in which considerable innovation is both possible and highly desirable. Companies that can master the art of developing the best analytical approach for their programs have the opportunity to reduce costs but also reach the clinic and then the market more quickly. Armed with a competence such as this, firms can achieve higher revenues and a greater share of the market. Your analytical strategy could help your company achieve a competitive advantage.
About the author: Nick Hutchinson is a Technical Content Marketing Manager at Sartorius Stedim Biotech.
(ref 1) Shah, D.S.H., Wernhart, C. & Athey, D. (2016) Advanced Protein Engineering Enhances Biopharmaceutical Manufacturing and Analytics. Bioprocess International. 14(10) November 2016. Available online.
(ref 2) Matthias, K.H., Birchall, C., Lodge, A., Mysliwy, J., Thirlway, J. & Shah, D.S.H. (2016) A protein-engineering approach to in-process assay development during Ab-drug conjugate manufacture: a proof of concept with trastuzumab. Poster. World ADC, San Diego. 2016. Available online.