PharmAI – Industry is Smartening Up to Potential of Artificial Intelligence

Exploring Pharma Deal-Making For AI And Machine Learning Technologies
Executive Summary
AstraZeneca’s pact with the UK-based artificial intelligence leader BenevolentAI, in April 2019, is one component in a string of recent deals that have highlighted big pharma’s desire to embed AI and machine learning within the R&D process. In total, 13 such collaborations have been tracked in the past 24 months, according to the Strategic Transactions database.
Artificial Intelligence (AI) has enormous potential in changing the delivery of health care and improving patient outcomes, with applications in pattern detection, patient monitoring, disease diagnosis and treatment selection. The rationale is that AI can be used to enhance the decision-making process, taking into account far more data than are available to physicians via conventional means. The addition of machine learning (ML) algorithms enables the AI to become better over time, increasing the accuracy or timeliness of its recommendations. Recognizing this broad potential, the FDA has recently sought to spur the industry with its discussion paper on regulating Software as a Medical Device (SaMD) products.
The application of AI and ML techniques to the pharmaceutical industry will have less of a direct impact on patients, although may be fundamental to improving R&D productivity and sustaining the current pace of innovation. Leading pharma companies are almost universally detailing such digitalization initiatives in their investor-facing materials, showing that they are at the forefront of this vital industry trend.
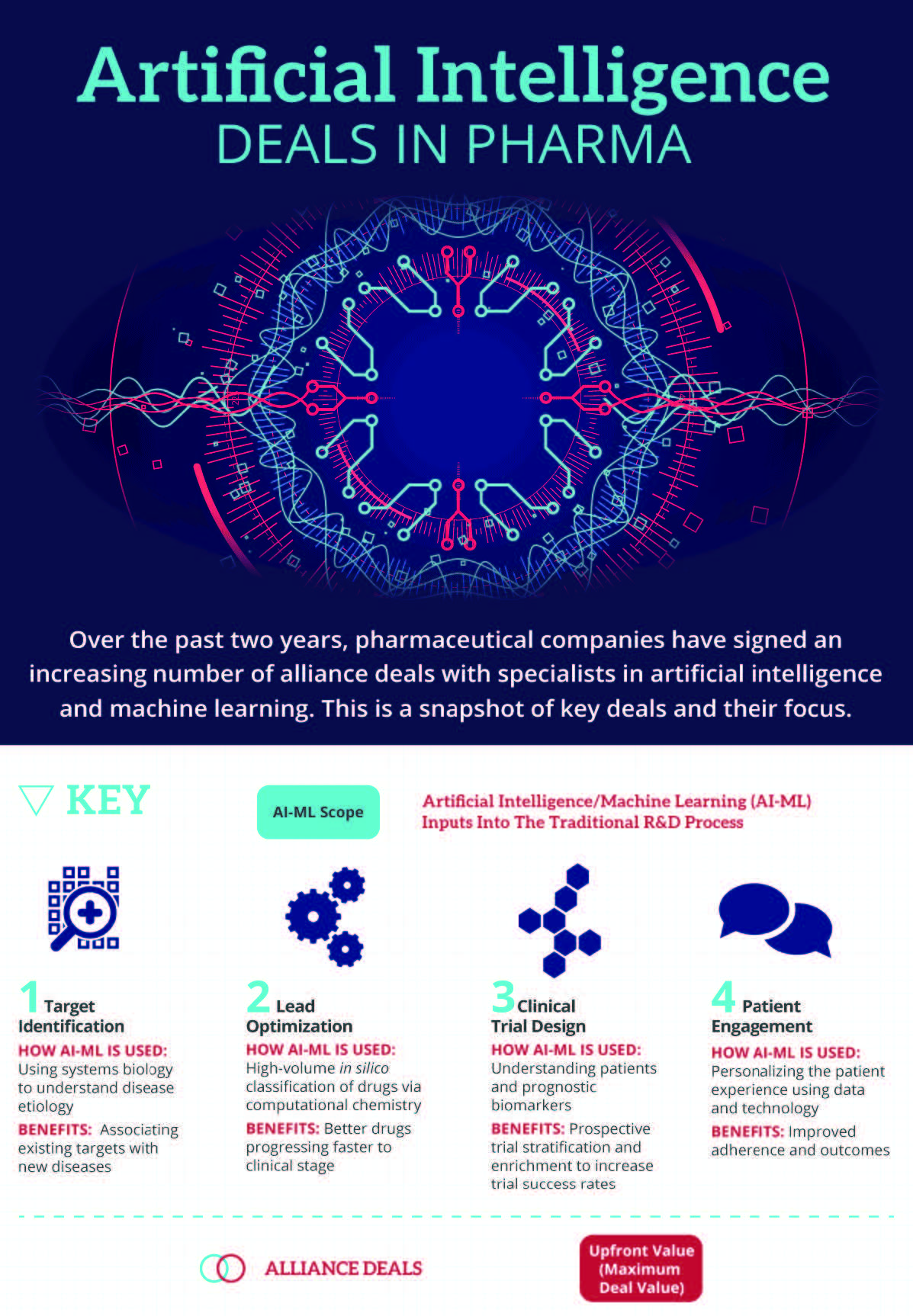
Pharma Companies Are Accelerating Adoption Of AI-ML Technologies
Deal-making between pharma and AI specialists is becoming increasingly commonplace, as the industry seeks external pioneers to validate the technology, in addition to building internal data science teams. Over the past two years, to Informa Pharma Intelligence’s Strategic Transactions has noted 13 such alliances with specific mention of the AI or ML learning capabilities that one partner brings. Of these, 10 deals have occurred in the past 12 months, pointing towards an acceleration in the adoption of such technologies (see Exhibit 1).
Among these deals are notable big pharma examples such as Roche, Pfizer Inc., AstraZeneca PLC, GlaxoSmithKline PLC, Bristol-Myers Squibb and Sanofi. The alliance between bluebird bio and Gritstone has the highest potential deal value, with milestone payments of up to $1.2bn, in addition to $20m upfront. Exscientia has now concluded several major deals, each with different pharmaceutical companies, showing that these platforms are being applied to individual discovery programs and are not being used in exclusive arrangements with a single licensee. Furthermore, the approach is not restricted to any particular therapeutic area, with examples spanning oncology, immunology, central nervous system disorders, cardiovascular diseases and metabolic disorders. The extent of collaboration is so far focused towards early preclinical research, such as target identification and lead optimization. Still, the potential for AI-ML is certainly broader.
Exhibit 1. Major Pharma-AI/ML Alliance Deals In The Past Two Years
 AI-ML As Means To Improve R&D Productivity
AI-ML As Means To Improve R&D Productivity
Many of the current deals between pharma and AI-ML experts have a specific R&D project in mind, aiming to bring better compounds into clinical development at a faster rate, thereby shortening development timelines and improving the eventual likelihood of approval. It may well be that better understanding of complex diseases will yield previously unknown drug targets – and potentially entirely new, differentiated breakthrough therapies. Nevertheless, the core business case behind pharma’s adoption of AI-ML techniques is as a productivity initiative, allowing pharma to realize a higher return on its R&D investment. With estimates for the cost to bring a new drug to market continuing to spiral upwards – the most recent calculation by Tufts Center for the Study of Drug Development places the figure at $2.6bn – anything that can reverse this trend is sorely needed. As AstraZeneca R&D chief Mene Pangalos simplified in an interview with Scrip: “It takes many years to get a candidate into the clinic so could you write an algorithm that could speed that process up and do it more efficiently?”
While AI-ML does not replace basic research into human biology and disease etiology, its great strength is that it is able to make connections within complex data sources that no human brain could realistically hope to make, without any prejudice. The intuition of scientists can be augmented by the processing of unfathomable amounts of information to arrive at the best solutions based on the data available. This allows an unbiased approach to the question at hand, with any new data and insights generated further strengthening the underlying AI-ML platform. This is immediately useful for diseases that are currently poorly defined or understood, potentially discovering new targets whose relationship to the disease state is not obvious. BenevolentAI itself has created a drug discovery program for amyotrophic lateral sclerosis based on a target previously evaluated in breast cancer.
AstraZeneca has pivoted the development of saracatinib away from hematological cancers and into idiopathic pulmonary fibrosis based on the insights of digital health and AI pioneer Joel Dudley at Mount Sinai in New York. The microbiome field is another rich resource for AI-derived insights, considering the innumerable interactions between human biology and the 10-100 trillion symbiotic micro-organisms within each of us, increasingly being recognized as an organ in its own right, or even our second genome.
Even with a sound scientific basis for a drug discovery project, the design and selection of the best drug candidate is limited by the resources available. There is a trade-off between the time spent on lead optimization and the need to progress a candidate into the clinic, which inevitably contributes to the failure of drugs due to unforeseen pharmacokinetic, toxicity or efficacy shortcomings. Existing knowledge around druglike properties and the target interaction can be distilled with AI into better drug candidates for preclinical testing. UK-based Exscientia claims to deliver clinical-stage drug candidates in one quarter of the time of traditional approaches, suggesting the reason for its popularity as a partner for pharmaceutical companies.
A drug’s success can hinge solely on the design of its clinical trial program, which is another area in which AI can help to plot the best course. A more complete understanding of the disease state, target, drug and patient characteristics can enable prospective patient stratification for treatment responders. This increases the likelihood of a favorable outcome in the clinical trial, or avoids the scenario in which expensive Phase III trials are repeated after the best design is only uncovered due to retrospective analyses. Even if a drug reaches the market, razorthin differences between competitors can be exaggerated by clinical trial design, yielding vastly different market prospects. Taking the current crop of programmed death-1 (PD-1) inhibitors, it is commonly asserted among prescribers that the drugs within the class are extremely similar. The correct choice of patient group, biomarker, background therapy or comparator arm in a pivotal clinical trial may potentially be worth many billions of dollars over the lifetime of the drug. While the advent of AI-ML may have come too late for the likes of Opdivo (nivolumab; Bristol-Myers Squibb/Ono Pharmaceutical) and Keytruda’s (pembrolizumab; Merck & Co) initial development, it can absolutely shape the next wave of immuno-oncology, matching non-responding patients to the most appropriate drug regimen based on biomarker data and known mechanisms of PD-1 inhibitor resistance. Pfizer instigated a collaboration with IBM in December 2016 with this in mind.
Reconciling The Hype Of AI-ML And Shortcomings in Drug Discovery
There is undoubtedly an element of “fear of missing out” driving the adoption of AI-ML that accompanies the uptick in deal-making lately. Early pioneers such as Exscientia and BenevolentAI are clearly striking the right chord with their technologies, marketing them as essential solutions for modern drug discovery. However, we are still yet to achieve proof-of-concept for an AI-enabled drug discovery program to result in an approved, successful product. And even when this does occur, it will be difficult to quantify the exact benefit that the AI approach added in terms of timelines, R&D spend, and clinical benefit, unless a lesser-informed, competing pharma company opts to become the placebo. It will only be with many approved examples that it would be possible to validate claims of better drugs and higher likelihood of approvals, but arguably AI-ML will come up short in terms of fixing the true bottleneck of drug discovery: translating preclinical research into clinical proof-of-concept at Phase II. The AI can only be as smart as our basic understanding of human biology allows, so drug discovery is always going to be an iterative process, with failures being a necessary component. Perhaps tellingly, IBM is now stopping sales of its Watson AI in the field of drug discovery, with previous reports that its application in oncology clinical practice has not been able to live up to IBM’s own lofty expectations. Regardless of how much AI-ML is actually able to deliver, if these technologies can help to better understand diseases, create fully optimized drug candidates and test them in patients in the smartest way possible, it would be negligent for pharma executives not to make use of its capabilities.
Learn more about how Artificial Intelligence is significantly influencing global healthcare and get hands-on experience with digital medicine products such as therapeutics, assessment tools, diagnostics, wearable tech and AI/analytical tools by attending a free session and reception during Digital Medicine & Medtech Showcase.
Register now to attend The impact of AI: How AI and ML are transforming R&D, and what's next to come at 4:00pm and the Experience Zone Exhibits Reception from 5:00pm to 6:00pm on Monday, January 13, 2020, at the Park 55 San Francisco – A Hilton Hotel.