By María de la Luz Lara Méndez, Regulatory Affairs Specialist LATAM & Adriana Hernández Trejo, Regulatory Affairs Specialist LATAM
Talking about global harmonization implies that all regulatory agencies agree upon developing, implementing, and adopting the same guidelines and technical standards focused on quality, safety, and efficacy, that would be eventually applied by each Regulatory Agencies on the respective countries. Such harmonization may generate increased effectiveness and decreased costs for every country.
This is an extract taken from What is standing in the way of global harmonisation? Part II: Industry Voices. Click here to read the full article.
There are several global initiatives intended to drive harmonization and development, such as: Asian-Pacific Economic Cooperation (APEC), International Council for Harmonization (ICH), The International Pharmaceutical Regulators Programme (IPRP), Pan American Network for Drug Regulatory Harmonization (PANDRH). However, their effort has not stood out as originally intended because there are different obstacles standing in the way of the global harmonization.
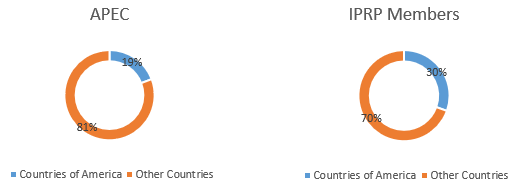
Which is the participation of the countries of America in those global initiatives?
- Figure 1. Asian-Pacific Economic Cooperation (2020) - https://www.apec.org/About-Us/About-APEC/Member-Economies
- Figure 2. The International Pharmaceutical Regulators Programme (2020) - http://www.iprp.global/members
- Figure 3. International Council for Harmonisation. Members (2020) - https://www.ich.org/page/members-observers
- Figure 4. International Council for Harmonisation. Observers (2020) - https://www.ich.org/page/members-observers
It is worth to mention that many Regulatory Agencies in America are recognized by the Pan American Health Organisation (PAHO) and the World Health Organisation (WHO) as they comply with international standards, such as the Food and Drug Administration (FDA) in the US, Health Canada, the Commission for Protection Against Sanitary Risks (COFEPRIS) in Mexico, the National Administration of Drugs, Food and Medical Technology (ANMAT) in Argentina, the National Agency of Sanitary Surveillance, Ministério da Saúde (ANVISA) in Brazil, the Institute of Public Health (ISP) in Chile, the National Institute for Drugs and Food Surveillance (INVIMA) in Colombia, and the Center for the State Control of Drug Quality, Public Health Ministry, in Cuba. Nevertheless, despite being recognized under international standards, if we compare their regulatory processes, they are far from being harmonized.
So, if harmonization initiatives exist, and the agencies look for a compliance under the same international standards and they have further adopted some guidelines in several countries, why this global harmonization has not occurred?
Several factors may be considered obstacles that represent a constraint for global harmonization. We consider the following as the most relevant:
- Political changes – A change of Federal Government brings about modifications within Regulatory Agencies, most frequently the Head of such agencies. In most Latin American countries, directive positions within the National Regulatory Agencies have become political positions instead of being of technical-scientific nature. These changes impinge on the actions taken by the former Head as new actions are proposed. This causes the delay and sometimes the termination of any global harmonization activities previously planned.
- Experience within the respective Agency – Several agencies do not have experts nor specialists in every subject. Thus, the introduction of new systems or technologies is frequently a time-consuming process, particularly in Latin American countries.
- Regional regulation – Each country possesses its regulatory framework and sometimes it is not updated according to the international guidelines because global harmonization is not contemplated by the political agenda of Presidents, Congresses, Senators, and Prime Ministers. This causes the stalling of transformation, innovation, and the capacity to improve public health.
- Collaboration among Agencies – In spite the collaborative work among Regulatory Agencies aimed towards harmonisation through different initiatives, they are frequently disrupted as a connection between them is not attained. Therefore, there is no collaboration excepting such initiative.
- Insufficient resources – Several regulatory agencies face resource constraints of different nature such as human, financial, and infrastructure. This lack of resources within institutions causes activity saturation resulting in reduced operative capacity for essential processes. Thus, other activities or projects different from daily activities are left aside.
- Technology – Due to the aforementioned lack of resources, the implementation of new technologies is deficient within the Agencies. This generates a low availability of information for the interested parties. In turn, this also affects the prompt advancement towards regulatory, technological, and technical harmonization for some agencies.
- Socioeconomic aspects – Another aspect that should be also considered regarding harmonization are the costs and fees established by each Agency to evaluate procedures and services. In many cases, this prompts the evaluation of unnecessary procedures causing an additional administrative load that prevents health professionals from developing and implementing optimal evaluation strategies aligned with international standards.
- National Identity – Frequently, the concept of national identity may be an obstacle for global harmonization as the acceptance of directives different from the internal initiative of the country it is often thought as an infringement or violation of the nationalist thinking. This has been caused by the lack of bilateral agreements that would allow a mutual acknowledgment.
In order to attain a harmonization, we consider that it is essential the involvement of several figures participating in all health-related processes, such as the pharmaceutical industry, Universities/Academia, and the authorities. In this way, it is possible to migrate towards a confidence-based framework with scientific value and the delegation of responsibilities among all the involved parties. Consequently, the agencies may carry out collaborative work with other counties based on technical, scientific, and regulatory aspects allowing the establishment of a global framework that is possible and feasible for all probable scenarios.
Finally, the questions we should be asking ourselves in this regard are why is necessary a global harmonization? and which are the advantages for each one of the countries involved? If we wish to answer these questions, an analysis must be performed considering the needs and conditions of each country. Additionally, it is necessary to raise interest and to drive the willingness to establish directives towards a global harmonization and to continually support this kind of initiatives.
This is an extract taken from What is standing in the way of global harmonisation? Part II: Industry Voices. Click here to read the full article.
References
1. “The need for global regulatory harmonization: A public health imperative,” Zerhouni, Elias and Margaret Hamburg. Science Translational Medicine. 8:338 (2016)
2. Regulatory Harmonization and convergence. https://www.fda.gov/vaccines-blood-biologics/international-activities/regulatory-harmonization-and-convergence
4. “Glocalization: Balancing Global and Local Concerns in Manufacturing and the Supply Chain”, Pharma´s Almanac. PAQ3 2019