Complying with trial registries and other global disclosure reporting requirements an ongoing challenge

Registering clinical trials globally, along with the ever-multiplying regulatory document landscape, can lead to untenable oversight and compliance issues.
Since the year 2000, approximately two new trial registries have opened each year. Thomas Wicks, head of Transparency Operations at Citeline, said, “These range from large, well-known registries like ClinicalTrials.gov, which hosts over half a million trials, to smaller ones like Nepal's registry with just 15 trials.”
Clinical trial registries are the basic part of the clinical trial disclosure and transparency bailiwick. The other parts come in the form of protocol synopsis, protocols, plain language protocol synopses (PLPS), recruitment information, summary results, results, plain language study summaries (PLSS), informed consent forms, and even clinical study reports, before entering the regulatory authorization stage.
Wicks spent time telling the Clinical Data Disclosure Europe conference audience about the many requirements and challenges of keeping track of these documents, as well as potential penalties for non-compliance.
Wicks said there are 59 registries and over 100 countries that have some kind of requirement or description of what they want to do with disclosure. On the registries side, many of are voluntary, or quasi voluntary, where there is no regulation, but the ethics committees require it, or because it's just the right thing to do.
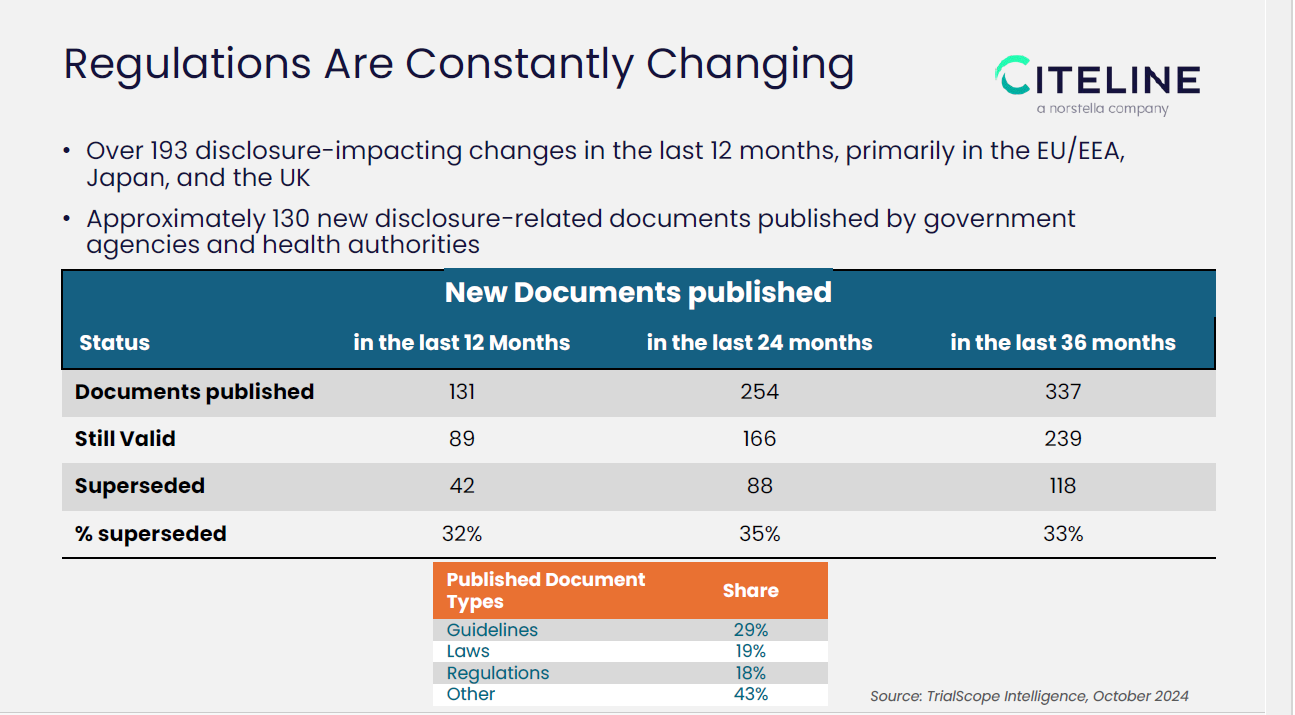 However, outside of the actual trial registries, as the slide above indicates, in just the past 12 months, 131 new documents related to clinical trial disclosure were published. Wicks said, “Even more striking is the rate at which these documents are updated or replaced. Almost a third of these documents are being replaced every year." Because companies rarely have a centralized staff to manage or track requirements, or have a CRO that is managing but maybe sponsors aren’t sure or providing oversight, Wicks noted this may not be the best practice for compliance.
However, outside of the actual trial registries, as the slide above indicates, in just the past 12 months, 131 new documents related to clinical trial disclosure were published. Wicks said, “Even more striking is the rate at which these documents are updated or replaced. Almost a third of these documents are being replaced every year." Because companies rarely have a centralized staff to manage or track requirements, or have a CRO that is managing but maybe sponsors aren’t sure or providing oversight, Wicks noted this may not be the best practice for compliance.
Non-compliance penalties in this area of disclosure and transparency range from financial penalties, public naming and shaming, trial suspension, market approval denial, or criminal sanctions. Financial penalties vary widely with starting fines ranging from $125 in Slovakia to over $35,000 in the Netherlands and up to $500,000 in Belgium.
Thomas warns, "These are potentially devastating penalties, even if they haven't been levied yet." He added that many in industry are waiting for that shoe to drop, however, “…most health authorities, are trying to work with sponsors and give them time to fix their problems.”
As trials progress through different stages, from approval to completion to marketing authorization, the disclosure requirements multiply. "You can imagine even a very modest portfolio six trials, and probably ridiculously small. But these data multiply," Wicks explains. He suggested that organizations consider the following best practices for disclosure management—proactive regulatory intelligence, centralized management, and internal audits.
Wicks will be presenting a step-by-step 100-day framework to evaluate your organization’s clinical trial disclosure maturity at our upcoming conference.