Kite Pharma’s cell therapy challenges: Manufacture, release, and distribution of products

As part of Cell and Gene Therapy Manufacturing Digital Week, Prentice Curry, Senior Vice President of Quality and Compliance at Kite Pharma, presented a webinar on the challenges in the manufacture, release, and distribution of cell therapy products. Here we pick out some of the key highlights. Watch the full webinar presentation, as well as seven other on-demand webinars, here.
‘Cell therapy products are showing promising clinical efficacy and now the first products are approved commercially in the United States and soon in Europe. These products are challenging to manufacture, test, and release as well as distribute safely back to the patient.’
In the course of his presentation, Prentice Curry explores these challenges and looks at how to build effective business processes to address them. For Curry, the main challenges include:
- Manufacturing Process
- Aseptic Processing
- Analytical Assays & Product Release by QP
- Logistics
- Traceability - ‘tracking the product to the patients…preventing mix-ups, where we are sure of the flow of the logistics chain of the patient’s blood samples through the manufacturing process’.
- Special Considerations and regulatory requirements
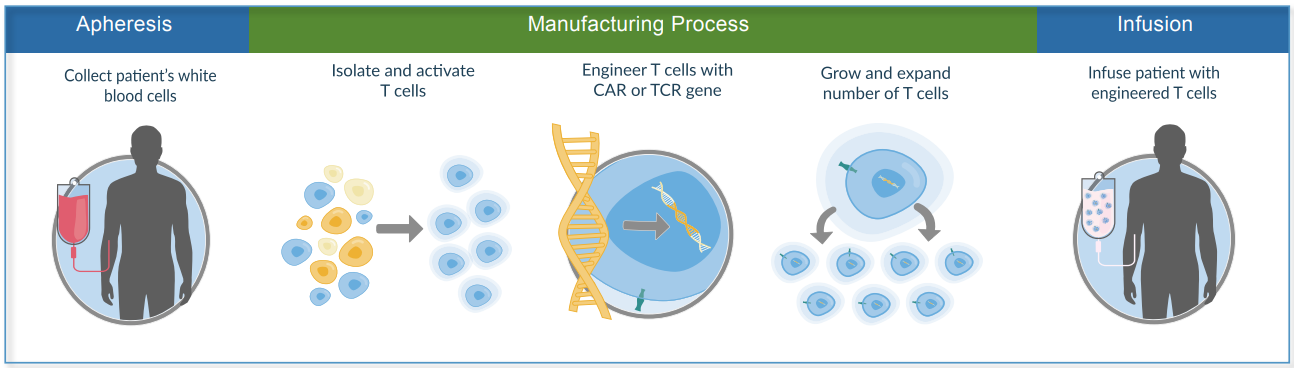
Using Kite’s leading commercial product – an engineered autologous cell therapy that harnesses the power of patients’ own T cells to treat lymphoma - as a case study, Curry explains how ‘T cell stimulation, growth and formulation are critical to an efficient manufacturing process, and that patients depend on robust and efficient manufacturing process.’ He elaborates on how they use a continuous process with no downstream purification steps.
One aspect of delivery that Curry focuses on is that patient need drives optimal lot release cycle time in Oncology, detailing how:
- Each patient lot release is targeted within days of manufacture
- Single patient dose manufactured and tested
- Rapid methods used
- Deviation management and QP disposition cycle time must be streamlined and adapted to meet patient needs
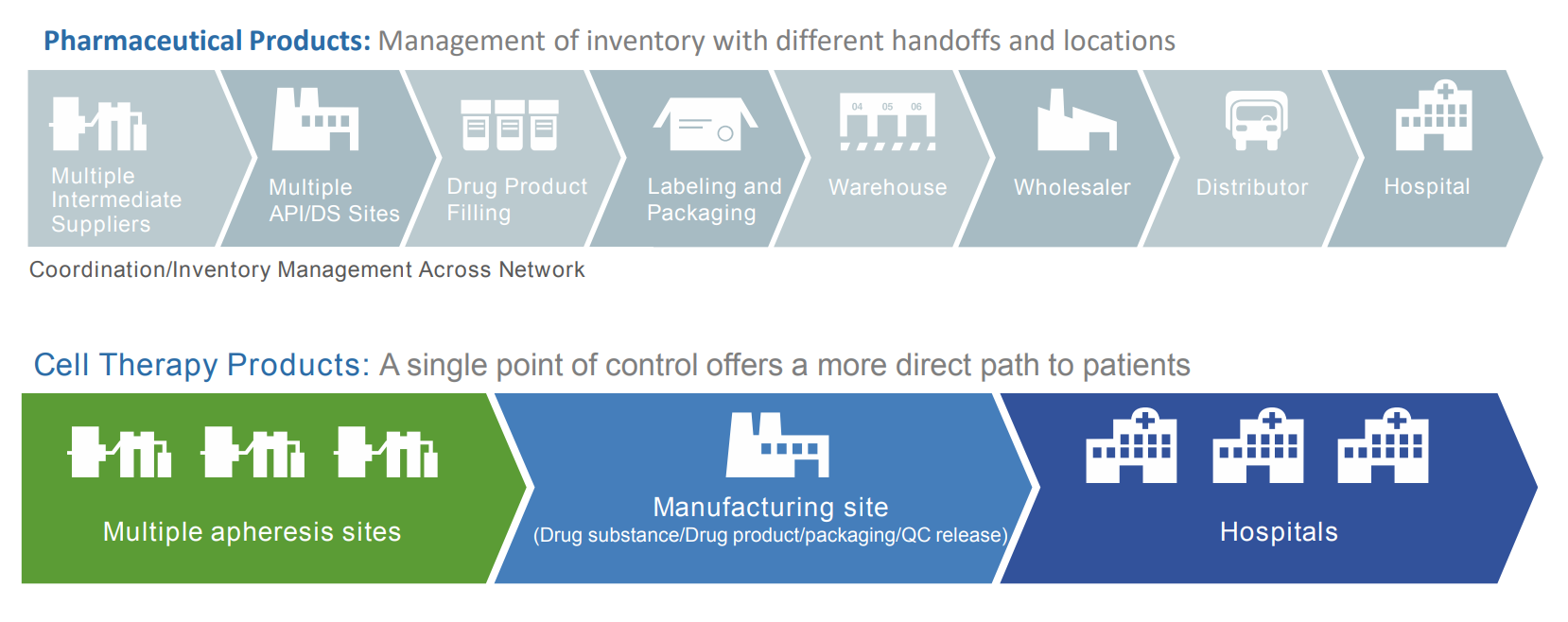
Pharma products vs cell therapy products
Curry emphasises that when it comes to cell therapies, ‘every day matters, leading to a direct path to patients’, especially when compared to a traditional pharma product:
‘A typical pharmaceutical product is a multi-step process that goes over weeks or months. You start with a number of suppliers that could be all over the world and you gather all these suppliers together to produce an API or drug substance. The drug product is then filled, often not even in the same facility and then all of this is shipped to a labelling and packaging center and then on to warehouses to then be distributed all round the world.’
In comparison, ‘a call therapy in many cases is much simpler with a simpler workflow. You need to cut quite a few of these steps out because of the direct path to the patient.’ The diagram below shows this difference in complexity.
CLICK HERE to watch the full 50-minute presentation, as well as seven other on-demand webinars from the 2018 Cell & Gene Therapy Manufacturing Digital Week.