REPORT: Antibody Therapeutic Modalities 2020

Within antibody engineering and therapeutics, there are now a number of different modalities the industry is working on, including monoclonal antibodies (mAbs), protein therapeutics, bispecifics, immuno-oncology checkpoints, antibody-drug conjugates (ADCs) and CAR-T/cellular therapies. At the same time, work on these modalities is increasingly exploring new non-cancer targets.
In May 2020, the Antibody Engineering & Therapeutics conference series conducted a study of industry professionals from around the world. This final report based on 166 survey responses reveals unique insights into the development of and approach to each of these novel modalities.
Explore or download the report by clicking on the image above, or here.
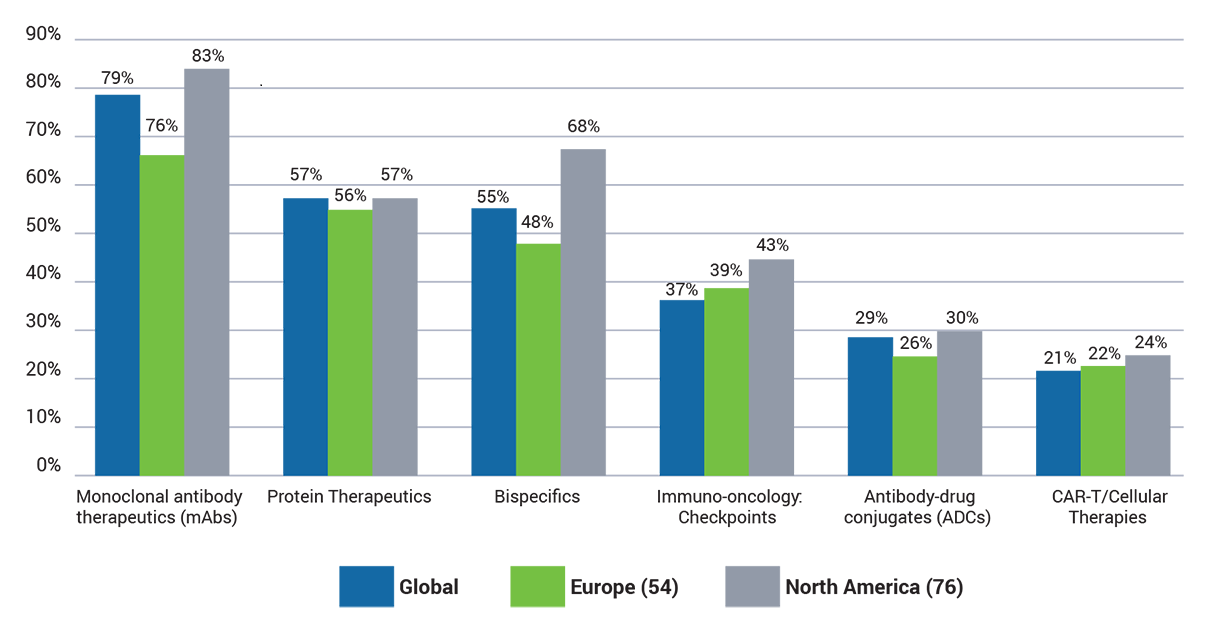
Which of the following types of therapeutic is your company currently working with?
Unsurprisingly, mAbs were the most common (79%) type of therapeutic that respondents are working on, followed by 57% of companies developing protein therapeutics and 55% bispecifics.
Over the course of this report, we explore what phase of development companies are in for each modality, what they are targeting, the challenges that still need to be overcome and the enabling technologies and platforms being used.
Key Insights
- 17% of respondents working on immuno-oncology checkpoints have therapies in Phase III or IV - the highest of any modality.
- 36% of respondents are working on therapeutics targeting auto-immune diseases - the highest target after cancer.
- 55% of respondents say that new targets/discovery approaches is the biggest challenge they are facing.
- 62% of respondents working with mAbs see computational approaches/Machine learning/Artificial Intelligence as the most promising technology for enabling development.