Viral vectors have biggest limitations in vector manufacturing, according to industry survey

Viral vectors used in production have the biggest limitations as starting materials for viral vector manufacturing, according to 55% cell and gene therapy professionals in a recent study by Informa Connect. Answers from the respondents, who were largely from biotech companies in North America and Europe, revealed the areas for improvement in the use of vectors across cell and gene therapy production.
Plasmids closely follow behind as the second most limiting raw material, selected by half of the respondents, in addition to cell lines (45%), media (26%) and serum (25%).
In the scenario of transitioning to commercial scale manufacturing of viral vectors, 61% of the cell and gene therapy professionals viewed the volume of supply to be the most critical pain point. The next in line was fairly evenly split between supplier qualification (39%), inefficiency in downstream processes (37%) and regulatory support (37%).
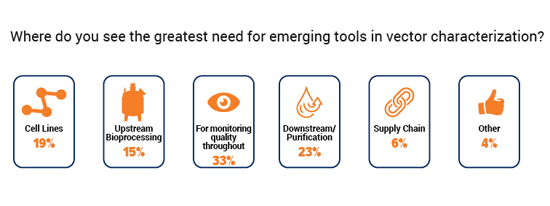 Overall quality monitoring was voted the greatest need for emerging tools in vector characterization. It was selected by a third of our respondents, whereas just under a quarter found downstream/purification to be the most urgent gap.
Overall quality monitoring was voted the greatest need for emerging tools in vector characterization. It was selected by a third of our respondents, whereas just under a quarter found downstream/purification to be the most urgent gap.