As part of Antibody Engineering & Therapeutics Digital Week, Christine E. Engeland, MD, PhD, Physician-Scientist, Departments of Medical Oncology and Translational Oncology at the National Center for Tumor Diseases (NCT), detailed the work that NCT has carried out in developing oncolytic vaccines using encoded bispecific T-cell engagers. Watch the full on-demand webinar here.
“Challenges in current cancer immunotherapy include increasing response rates and decreasing toxicity. We have developed tumor-selective oncolytic vectors for delivery of immunomodulators to avoid systemic exposure and mitigate toxicity. Furthermore, vector-mediated oncolysis serves as an in situ tumor vaccine, inducing synergistic anti-tumor immune responses. This talk highlights the versatility of our vector system and avenues for clinical translation.”
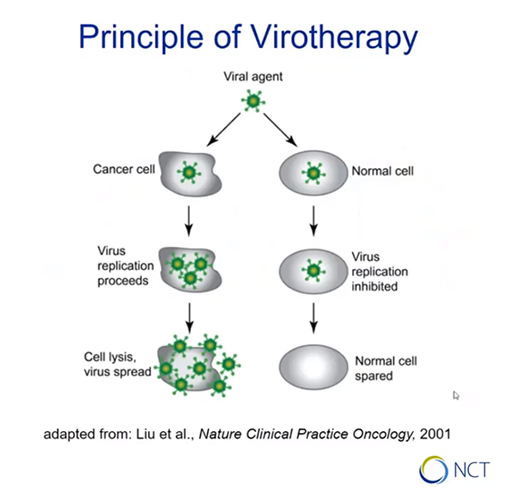
Oncolytic virotherapy
Is there a causal relationship between viral infection and tumor remission? Engeland presents the basic principle of virotherapy: certain viruses replicate selectively in malignant cells, leading to cancer cell lysis. If normal cells are infected with the same virus, replication is not affected and they are spared from lysis.
RELATED ARTICLE: The emergence and benefits of bispecific antibodies
Targeting tumors with oncolytic viruses
Engeland explains that with this principle in mind, the team at NCT came up with a hypothesis: if they could encode bispecific T cell engagers (BiTEs) within the oncolytic virus vector, they could achieve local delivery to tumor sites and potentially synergistic effects with the oncolytic virus, and also tackle some of the challenges for BiTEs in solid tumors that are delivering toxicity.
Starting off the project, the team designed measles virus vectors encoding BiTEs, targeting murine and human CD3 and using CD20 or CD8 as model tumor antigens. After generating viral particles from the vectors, tests were carried out to investigate their efficacy on patient-derived tumor cultures and mouse models.
Can this therapy work in humans?
After a series of tests, NCT have now developed viral vectors encoding immune checkpoint inhibitors such as antibodies against PD-1 or PD-L1. This method had found benefits in mice with pancreatic and colorectal cancers compared to results from using checkpoint blockade immunotherapy or the measles virus alone.
The study is now entering Phase I/II clinical trials of the oncolytic measles vaccines combined with anti-PD-1. It will be accompanied by a translational research program in which serial biopsies from enrolled patients will be comprehensively analysed to find out whether the results previously seen in mice can be replicated.